Table of contents
Key Takeaways
- CD34+ humanized mice enable better predictions for therapies, reducing the translational gap between humans and mice.
- High-quality CD34+ cells from CGT Global enhance engraftment success rates for HIS mice in preclinical studies.
- Cell viability, purity, and accurate counts are crucial for consistent and predictive data from these models.
- Ordering bulk CD34+ cells can significantly reduce costs and improve experimental workflows for researchers.
- CGT Global offers customized solutions and resources to support scientists in their CD34+ cell sourcing needs.
Table of Contents
CD34+ Humanized Immune System Mice
MNC Cell Quality Assessment: Flow Cytometry Data from CGT Global
Cell Viability
Cell Purity
Cell Count
Sources of CD34+ Cells: Comparison Table for Bone Marrow and Cord Blood
Bulk Isolated CD34+ for Humanized Mice
About CGT Global and Additional Resources
Frequently Asked Questions
List of Abbreviations
The Importance of Mice Humanized with Primary CD34+ Cells
There is an urgent need for better therapies to treat individuals with life-limiting conditions. Yet, developing new medicines is constrained by high attrition rates, high cost, and long timelines to bring a drug from discovery to market approval. For this reason, improving the ability to predict the success of novel therapies in real-world human populations is critical.
Narrowing the human-to-mouse translational gap are Humanized Immune System (HIS) mice. HIS mice engrafted with primary CD34+ hematopoietic stem and progenitor cells (HSPCs) contain all human hematopoietic lineages, albeit to varying degrees. They recapitulate the complexities of the human immune system more effectively than wild-type mouse models. These advanced preclinical models are used in early-stage drug development to investigate the mechanism of action, efficacy, and safety of new treatments such as immunotherapies.
Optimizing CD34+ cells for immunology research is critical in the large-scale generation of HIS mice for preclinical studies and poses significant technical challenges and is critical for producing consistent, predictive, and human-relevant data.
Successful engraftment of HIS mice starts with high-quality starting material. For investigators developing advanced therapies, achieving a statistically powered cohort of HIS mice requires not only quality but also a sufficient yield of CD34+ HSPCs.
At CGT Global, we support scientists generating HIS mice with the industry’s highest engraftment rates by delivering large, high-quality, single donor lots of isolated CD34+ cells from umbilical cord blood and bone marrow.
When you order CD34+ primary cells in bulk, you can save your lab a significant amount of time and money. See how bulk and inventory prices compare and how choosing bulk isolated cells can provide scientists with up to 80% cost savings by requesting our free Bulk vs. Inventory: Cost Comparison Resource.
In this blog, we show quality control data from CD34+ cells collected and processed in CGT Global’s Folsom, California laboratory, review the differences between CD34+ cells from cord blood and bone marrow, and answer some common questions about using bulk-isolated primary CD34+ cells to generate HIS mice at scale.
The Data Speaks for Itself
With CellsExpress, our lab experts perform bulk isolations to yield high volumes of CD34+ HSPC from umbilical cord blood and bone marrow. Using flow cytometry, the isolated CD34+ HSPC are characterized prior to cryopreservation to ensure
- High viability: percentage of live cells (≥ 80% post-thaw viability)
- High purity: percentage of cells in sample that are CD34+ positive (≥ 90% purity)
- Accurate cell count: guaranteed number of cells per vial
Table 1 shows a summary of quality control data for a sample of CD34+ cells isolated from cord blood and bone marrow in CGT Global’s Folsom, CA, USA laboratory.
Table 1: CD34+ cells from Cord Blood & Bone Marrow: Cell Count, Viability, and Purity
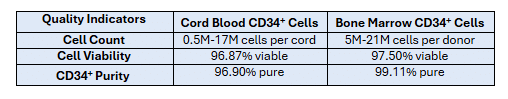
Below, we present more data and details for each cell quality parameter for bone marrow and cord blood CD34+ primary cells, beginning with cell viability.
Key Quality Parameter #1: Cell Viability
- Highly viable cells are alive and have high biological activity.
- Injecting dead cells can lead to adverse effects such as inflammation and undesirable immune responses in mice, potentially compromising the model and data.
- Cell viability is assessed using DAPI (4′,6-diamidino-2-phenylindole) fluorescent staining and flow cytometry analysis. Viable cells with intact membranes don’t uptake the dye. DAPI binds to the A-T regions of DNA in dead cells with compromised membranes leading to a signal detectable by flow cytometry lasers.
- Pre-cryopreservation, cells must be >95% viable to meet quality standards.
- Each product has a guaranteed ≥ 80% post-thaw viability if thawed using our validated protocol
Quality Control Data: CD34+ Cell Viability
Figure 1 shows measured cell viability data visualized in flow cytometry dot plots of CD34+ HSPC bulk-isolated from a (A) bone marrow (B) cord blood.
Bone Marrow
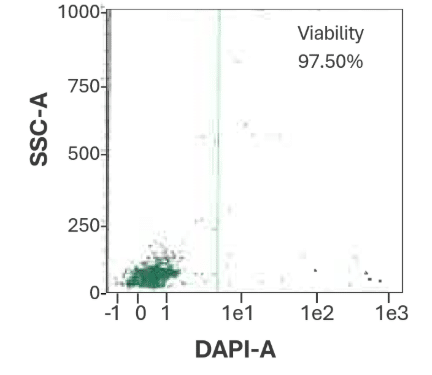
Figure 1A. CD34⁺ cell viability from bone marrow is 97.50%.
Representative flow cytometry dot plot showing viability of bulk-isolated human CD34⁺ cells from bone marrow.
Viability was assessed using DAPI staining and flow cytometric analysis on a MACSQuant® Analyzer.
Data from CGT Global’s Folsom, California, USA laboratory.
Cord Blood
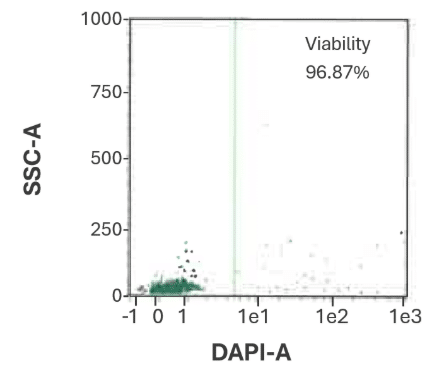
Figure 1B. CD34⁺ cell viability from cord blood is 96.87%.
Representative flow cytometry dot plot showing viability of bulk-isolated human CD34⁺ cells from cord blood.
Viability was assessed using DAPI staining and flow cytometric analysis on a MACSQuant® Analyzer.
Data from CGT Global’s Folsom, California, USA laboratory.
Key Quality Parameter #2: Cell Purity
- The purity of the sample calculates the percentage of cells in that sample that belong to the cell type of interest.
- Purity is important because specimens with low purity risk introducing unaccounted-for variables and inconsistent cell dosages.
- Cell purity is analyzed by labeling cells with an anti-CD34+ antibody conjugated to allophycocyanin (APC) and performing flow cytometry analysis to measure the fluorescence intensity.
- Each product is guaranteed to have ≥ 90% purity.
Quality Control Data: CD34+ Cell Purity
Figure 2 shows measured cell purity data visualized in flow cytometry dot plots of CD34+ HSPC bulk-isolated from a (A) bone marrow (B) cord blood.
Bone Marrow
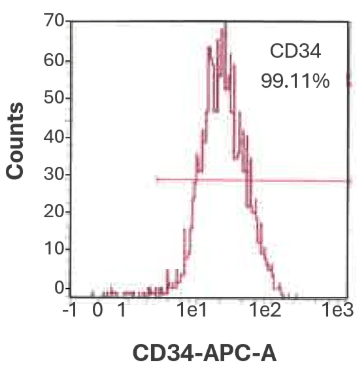
Figure 2A. CD34⁺ cell purity from bone marrow is 99.11%.
Representative flow cytometry histogram showing the purity of bulk-isolated human CD34⁺ cells from bone marrow.
Purity was assessed using CD34-APC antibody staining and flow cytometric analysis on a MACSQuant® Analyzer.
Data from CGT Global’s Folsom, California, USA laboratory.
Cord Blood
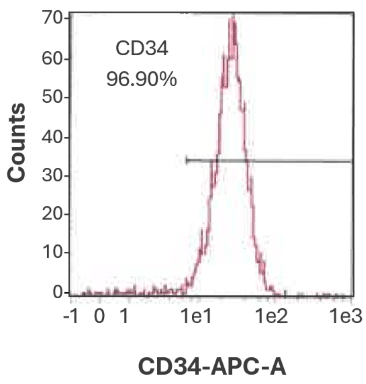
Figure 2B. CD34⁺ cell purity from cord blood is 96.90%.
Representative flow cytometry histogram showing the purity of bulk-isolated human CD34⁺ cells from cord blood.
Purity was assessed using CD34-APC antibody staining and flow cytometric analysis on a MACSQuant® Analyzer.
Data from CGT Global’s Folsom, California, USA laboratory.
Key Quality Parameter #3: Cell Count
- The cell count is the number of cells total per donor or how many cells are aliquoted in each vial.
- An accurate cell count supports the success of downstream experiments while an inaccurate cell count can lead to troubleshooting unexpected results.
- Cell counts are measured in duplicate using the automated NucleoCounter.
- We guarantee the cell count on every product.
- Cell counts per vial can be customized to fit your workflow.
- Cell counts displayed below are representative. Real world total cell counts may vary due to donor-to-donor variation.
Quality Control Data: CD34+ Cell Counts
CD34+ HSPC from Bone Marrow and Cord Blood Cell Counts
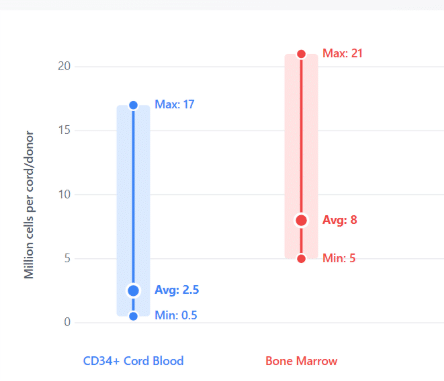
Figure 3. Cell counts for CD34+ cells isolated from bone marrow and cord blood.
Bone marrow donors yielded a minimum of 5 million, an average of 8 million, and a maximum of 21 million CD34+ cells.
Cord blood donors yielded a minimum of 0.5 million, an average of 2.5 million, and a maximum of 17 million CD34+ cells.
Data generated at CGT Global’s Folsom, California, USA laboratory. Actual cell counts may vary by donor.
Figure 3 shows minimum, average, and maximum total cell counts for CD34+ cells isolated from bone marrow and cord blood.
Isolated from bone marrow and cord blood, bulk CD34+ human hematopoietic cells for research use from CGT Global are highly viable, highly pure, and accurately counted.
The data demonstrate that these cells are a reliable source of high-quality CD34+ cells for humanized mice, helping to maximize engraftment success rates.
Best Source for CD34+ Primary Cells
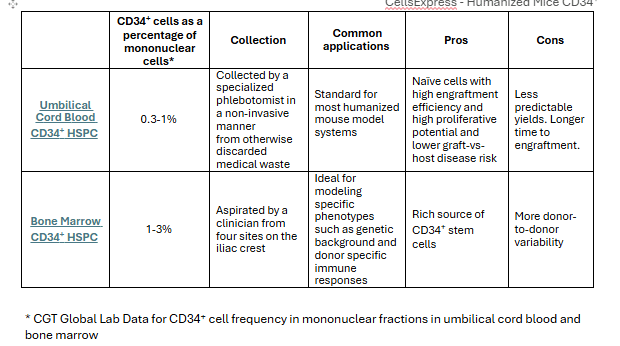
Humanized mice engrafted with CD34+ hematopoietic stem and progenitor cells (HSPCs) are valuable models for testing novel cell and gene therapies. Depending on your experimental design, cord blood or bone marrow may be the preferred source of CD34+ cells. See table 2 below for more information on CD34+ cells from different sources.
Table 2. Human umbilical cord blood and bone marrow sourced CD34+ cell frequency, collection methods, common applications, advantages, and disadvantages
Frequency of Actively Dividing CD34+ HSPC Differs Depending on the Cell Source
One study classified CD34⁺ hematopoietic stem and progenitor cells (HSPCs) from cord blood and bone marrow at baseline and found that bone marrow–derived CD34⁺ HSPCs were more likely to be in the S and G2/M phases of the cell cycle than CD34⁺ HSPCs derived from cord blood. After 48 hours of stimulation with stem cell factor (SCF) and interleukin-3 (IL-3), both cell sources exhibited similar levels of cell division.
Table 3: Comparison of proliferation at baseline and after 48-hour SCF + IL-3 stimulation of CD34⁺ stem cells from bone marrow and cord blood*

Results shown as mean ± standard error of mean
*Adapted from: De Bruyn, C., Delforge, A., Lagneaux, L., and Bron, D. (2000). Characterization of CD34+ subsets derived from bone marrow, umbilical cord blood and mobilized peripheral blood after stem cell factor and interleukin 3 stimulation. Bone Marrow Transplant. 25, 377–383. 10.1038/sj.bmt.1702145.
These results suggest that, at baseline, cord blood CD34+ HSPCs are more quiescent than bone marrow CD34+ HSPCs, which are more likely to be dividing. There was a significant increase in proliferation from baseline to 48 hours with SCF + IL-3 with cord blood cells appearing to be more responsive to stimulatory cues. When stimulated, cells from both sources had comparable frequencies of the cell population undergoing cell division. Interestingly, upon further categorization of the less differentiated CD34+CD38- cells, researchers reported that bone marrow–derived CD34+ cells did not demonstrate a significant increase in cell division as a result of growth factor and cytokine stimulation (5.6 ± 1.2% at day 0 vs. 8.6 ± 1.0% after 48 h) whereas the cord blood–derived cells did (2.0 ± 0.4% at day 0 vs. 19.2 ± 2.2% after 48 h).
This data is consistent with the notion that cord blood CD34+ HSPCs may take longer to engraft in HIS mice, but have higher longer-term engraftment potential.
These factors must be taken into account when you buy CD34+ primary cells so you can choose the best source for your specific mouse model and experimental design.
Humanized Immune System (HIS) mice have been foundational to key discoveries about how the human immune system can be harnessed to treat HIV and cancer. These models rely on CD34+ cells, the starting point for human-in-mouse hematopoiesis, which is a rare and precious resource in biomedical research.
By collecting, isolating, and engrafting these cells into mice, researchers have made important strides in developing immunotherapies, including immune checkpoint inhibitors and CAR-T therapies, for difficult-to-treat diseases. For example, scientists using CD34+ stem cells for xenograft models are finding new approaches to more personalized cancer treatments.
At CGT Global, we go above and beyond to provide scientists with the highest quality CD34+ hematopoietic stem cells for humanized mice leading to reliable engraftment rates in HIS mice.
As a premium CD34+ cell bulk supplier, we offer high yield isolations of highly viable and highly pure CD34+ cell populations to optimize humanized mouse development and accelerate the development of new therapeutics.
Necessary for reproducible results, large single donor lots of CD34+ are easy to secure with CGT Global’s CellsExpress services. Explore the resources below to learn how CellsExpress bulk isolations can reduce costs by up to 80% per vial compared to purchasing from inventory.
Resources
Learn more about CellsExpress for humanized mice and what we have to offer below
- For more information on cost savings for Bulk vs. Inventory, submit your email here.
- To learn about our Trial Vial and Recallable Donors, contact us here.
- Click here for our validated thawing protocol.
To review more data from our laboratory on CD34+ cells, submit your email and our team will be in touch.
[insert contact form link for redacted lab data]
About Us
Founded in 2010 by Cate Spears, CGT (Cell and Gene Therapy) Global is on a mission to transform the life sciences and medical industries.
As a leading global provider of human stem cells, primary immune cells, Leukopaks, bone marrow, cord blood, peripheral blood, and disease-state products, CGT Global expedites research, accelerates clinical trials, streamlines the commercialization process, and broadens patient access to life-changing therapies.
CGT Global is headquartered in Folsom, California, with a major shipping hub in Reno, Nevada, and 3 FDA- and CLIA-certified Cell Collection Centers in the United States (Folsom, CA; East Norriton, Pennsylvania; and Boston, Massachusetts). At each location, high-quality RUO and GMP biospecimens and cells are expertly collected and immediately processed in state-of-the-art laboratories. All domestic and international shipments are backed by a 100% satisfaction guarantee.
Ready to partner with an experienced collaborator for your cell sourcing needs?
Contact CGT Global Today to Accelerate Your Humanized Immune System Mouse Model Program
Phone: 530.303.3828 | 888.415.4215
Email: sales@cgt.global | Social: CGT Global’s LinkedIn
Written by: Joanna Wirkus, PhD
Frequently Asked Questions
- What are common applications of mice engrafted with human CD34+ HSPCs?
Preclinical oncology research often utilizes humanized immune system mice for immunotherapeutic drug discovery and testing personalized drug responses in patient-derived xenograft models. Humanized mice are also a good model to study in vivo stem cell gene delivery, CAR-T, and CAR-NK cell therapies.
Xu, X., et al. (2022). Large-cohort humanized NPI mice reconstituted with CD34+ hematopoietic stem cells are feasible for evaluating preclinical cancer immunotherapy. FASEB J
Chuprin, J., et al. (2023). Humanized mouse models for immuno-oncology research. Nat. Rev. Clin.
Jin, K.-T., et al. (2021). Development of humanized mouse with patient-derived xenografts for cancer immunotherapy studies: A comprehensive review. Cancer Sci.
Frecha, C., et al. (2011). In vivo gene delivery into hCD34+ cells in a humanized mouse model. Methods Mol. Biol
Mhaidly, R., et al. (2020). Humanized mice are precious tools for preclinical evaluation of CAR T and CAR NK cell therapies. Cancers (Basel)
2. Do you offer customization options for bulk-isolated CD34+ HSPC cells?
Yes. On all our products, we offer our clients made-to-order customization options, including vial size, cell counts per vial, isolation method (positive vs negative), anti-coagulants, fresh vs frozen, freezing media, shipping and packaging options, among others. Ask our team about custom solutions for specific needs.
3. How do I know the cells I purchase will work in my mouse model?
CGT Global offers a trial vial program so researchers can evaluate the performance of the cells in their model before they purchase in bulk.
Learn more about our trial vial program here.
4. What regulatory protections are in place?
Each collection center and laboratory complies with Good Laboratory Practice (GLP) regulations. All donations comply with federal regulation 21 CFR Part 1271 and applicable local laws. Donors are ethically consented using Institutional Review Board (IRB)-approved consent forms and protocols, and systems compliant with the Health Insurance Portability and Accountability Act (HIPAA). Our cell processing and isolation laboratories are registered with the Food and Drug Administration (FDA) and certified under the Clinical Laboratory Improvement Amendments (CLIA). We follow validated Standard Operating Procedures (SOPs) governed by a Quality Management System (QMS).
Click the links to learn more about how CGT Global ensures the responsible sourcing of human cells and our commitment to maintaining high standards and outlined in our quality policy.
5. Who are the donors?
Cord blood donors: Mothers provide consent,using IRB-approved forms, prior to delivery to donate their placenta and umbilical cord to biomedical research. This process is supported by trained cord blood collection technicians who are available in the labor and delivery unit 24/7, 365 days a year.
Bone marrow donors: We maintain one of the industry’s largest, most diverse, and prescreened donor pools, with recallable and repeat donors from coast to coast. Donor recruitment is often tailored to meet the specific needs of each project, including targeted demographics or disease states. More information about recallable donors can be found in this downloadable resource.
Learn more about Donor Recruitment and Attributes on our website.
6. What information do you provide about the donors?
Standard information includes:
- Demographics:
- Age
- Gender
- Ethnicity
- Smoking status
- Infectious disease screening includes (all negative):
- Hepatitis B
- Hepatitis C
- Human Immunodeficiency Virus types 1 and 2 (HIV1/2)
Additional information is available upon request such as:
- Body Mass Index (BMI)
- Medication
- Vaccination history
- Blood/genetic testing:
- Human Leukocyte Antigen (HLA)
- ABO blood group system
- Rhesus D (RhD) antigen typing
Additional infectious disease screening is available upon request: (e.g., Cytomegalovirus (CMV), Lymphocytic Choriomeningitis Virus (LCMV), etc.).
6. How are cells shipped?
Frozen cells are shipped in vapor-phase liquid nitrogen or on dry ice.
8. How quickly can you deliver to domestic locations?
Same-day and overnight shipments are coordinated from our East & West Coast Collection sites. Some locations qualify for same day delivery.
9. Do you ship internationally?
Yes, we ship cells worldwide and provide full customs and documentation support using FedEx and specialty couriers. The East & West Coast Collection sites are strategically located near major international airports. Shipments can include a data logger that records temperature every minute, ensuring traceable quality and confirming cell viability upon arrival. Packages are received at the airport by a courier and delivered directly to the client to prevent delays and expedite the delivery.
More information about shipping can be found at Shipping – CGT Global
10. What other factors may inform choosing cord blood or bone marrow derived cells?
Local regulations can determine how scientists source cells for their research. For example, France prohibits the use of human cells obtained from compensated donors. In such cases, cord blood-derived cells—typically donated without compensation—may be the preferred option.
11. How are the CD34+ HSPC obtained and isolated?
Umbilical cord blood is collected by trained medical staff immediately after birth. Partner hospitals are located near our cell isolation laboratories, allowing CD34+ HSPC to be isolated within 24 hours to ensure optimal cell viability.
Bone marrow is aspirated by a clinician from the iliac crest and the CD34+ HSPC cells are isolated immediately after collection.
The CGT Global laboratory uses Magnetic-Activated Cell Sorting (MACS).
CD34+ HSPCs are positively selected using immunomagnetic anti-CD34 (clone QBEND10) microbeads from the mononuclear cell fraction. See our behind the scenes blog to read about CD34+ isolation at CGT Global’s laboratory.
12. How is the quality of the CD34+ cells measured?
Isolated cells are characterized by flow cytometry prior to cryopreservation to ensure a high purity and high viability cell population. Each vial includes overfill to guarantee cell counts. Cells are guaranteed ≥ 80% post-thaw viability and ≥ 90% purity.
More information about CGT Global’s quality policy is available on our website.
13. What are the benefits of buying pre-isolated CD34+ HSPC cells?
By minimizing the time between cord blood and bone marrow collection and CD34+ HSPC cell isolation, we offer the highest-viability cells in the industry.
Purchasing pre-isolated cells instead of raw materials (whole cord blood or bone marrow) allows researchers to reallocate valuable resources toward collecting more data, analyzing results, and planning follow-up experiments.
Cell isolations can be tedious, time-consuming, and may require troubleshooting. Our lab experts are highly skilled in performing these isolations.
By outsourcing this labor to an experienced collaborator, researchers can reallocate or reduce staff time and equipment use, as well as consumables and biohazardous waste, needed for day-long cell isolations.
Within the context of large, humanized mouse model programs, unexpected logistical delays and technical troubleshooting related to cell count, viability, or purity can set back the experimental timeline. Establishing a reliable supply chain of consistent, high-quality cellular starting material de-risks operational workflow and supports the development of a successful, scalable, and sustainable humanized mouse model program.
14. What are the benefits of buying CD34+ HSPC cells in bulk?
Buying the maximum number of cells that can be isolated from a donor can result in cost savings of up to 80%. Click here to review our cost comparison resource to see how inventory prices compare to bulk. Researchers save time and money not only on starting materials, shipping, and logistics, but also by significantly reducing the need for internal resources—such as reagents, consumables, biohazardous waste disposal, and staff or equipment time. Biobanking large quantities of single-lot cells gives scientists greater flexibility to start experiments without waiting for shipments to arrive. Cryopreserved cells can remain viable after storage at the proper temperature for up to 7 years if they are thawed using our validated protocol. This type of inventory is a valuable investment for scientists considering rising costs of labor and inevitable inflation.
15. Do you offer fresh and frozen cells?
Yes. Many scientists prefer cryopreserved cells for the flexibility to start projects on their own timeline. However, because some loss of viability is expected as a result of the freeze-thaw process, scientists with the logistical capability to receive fresh shipments may opt for fresh cells instead, which are delivered at 4°C.
16. Do you offer CD34+ HSPC cells from sources other than cord blood and bone marrow?
Yes, we also offer CD34+ HSPCs isolated from mobilized peripheral blood depending on the project needs and desired yield. Phenotypically, they are more similar to bone marrow CD34+ HSPCs.
17. Why choose CGT Global?
All orders are backed by a 100% satisfaction guarantee.
List of Abbreviations
≥: Greater than or equal to BMI: Body Mass Index CAR-NK: Chimeric Antigen Receptor Natural Killer Cell CAR-T: Chimeric Antigen Receptor T Cell CD34+: Cluster of Differentiation 34 Positive CGT: Cell and Gene Therapy CLIA: Clinical Laboratory Improvement Amendments CMV: Cytomegalovirus FDA: Food and Drug Administration G2/M: G2 and Mitosis phases of the cell cycle HIS: Humanized Immune System HIV: Human Immunodeficiency Virus HLA: Human Leukocyte Antigen HSPC(s): Hematopoietic Stem and Progenitor Cell(s) h: Hour IL-3: Interleukin-3 IRB: Institutional Review Board LCMV: Lymphocytic Choriomeningitis Virus MACS: Magnetic-Activated Cell Sorting PDX: Patient-Derived Xenograft QMS: Quality Management System RhD: Rhesus D Antigen S phase: Synthesis phase of the cell cycle SCF: Stem Cell Factor SOP: Standard Operating Procedure