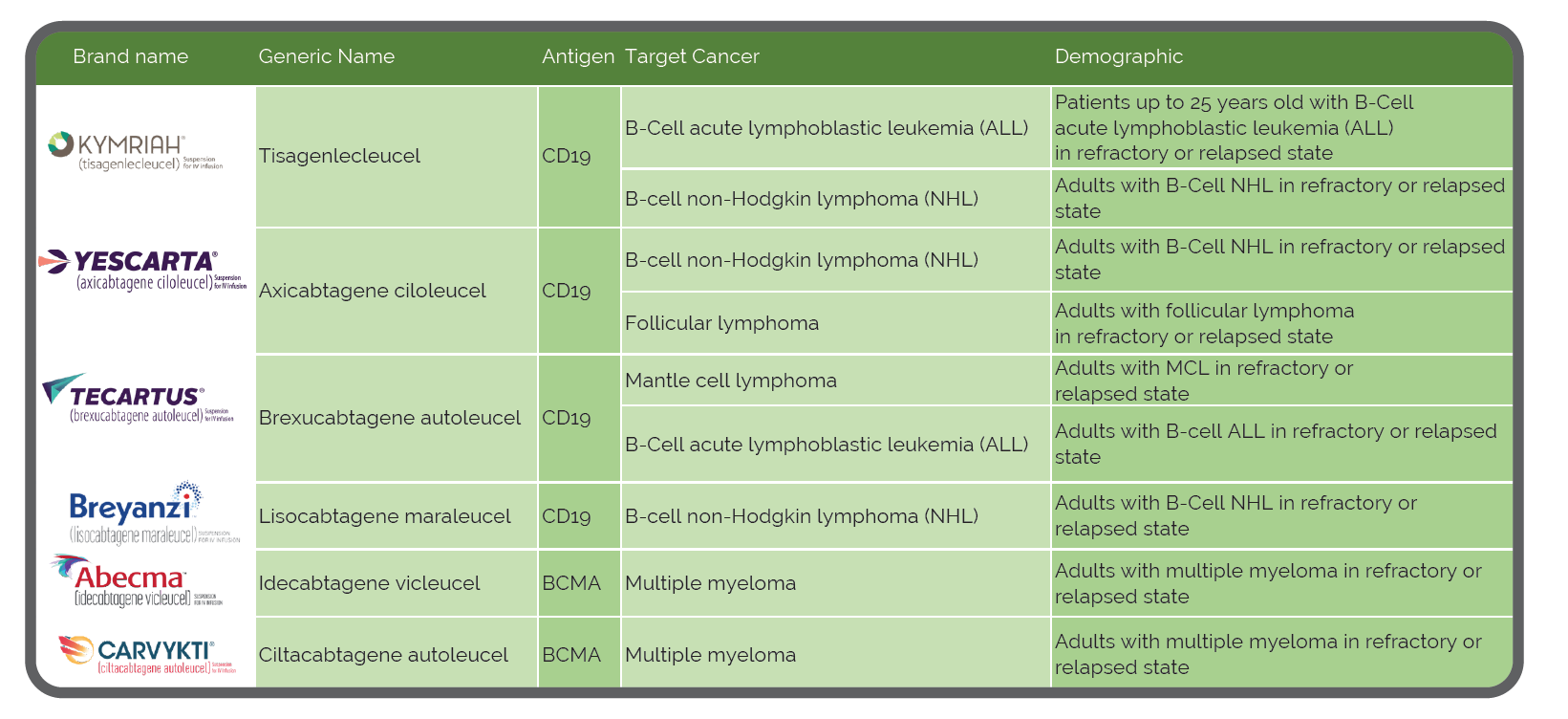
What is CAR-T Therapy?
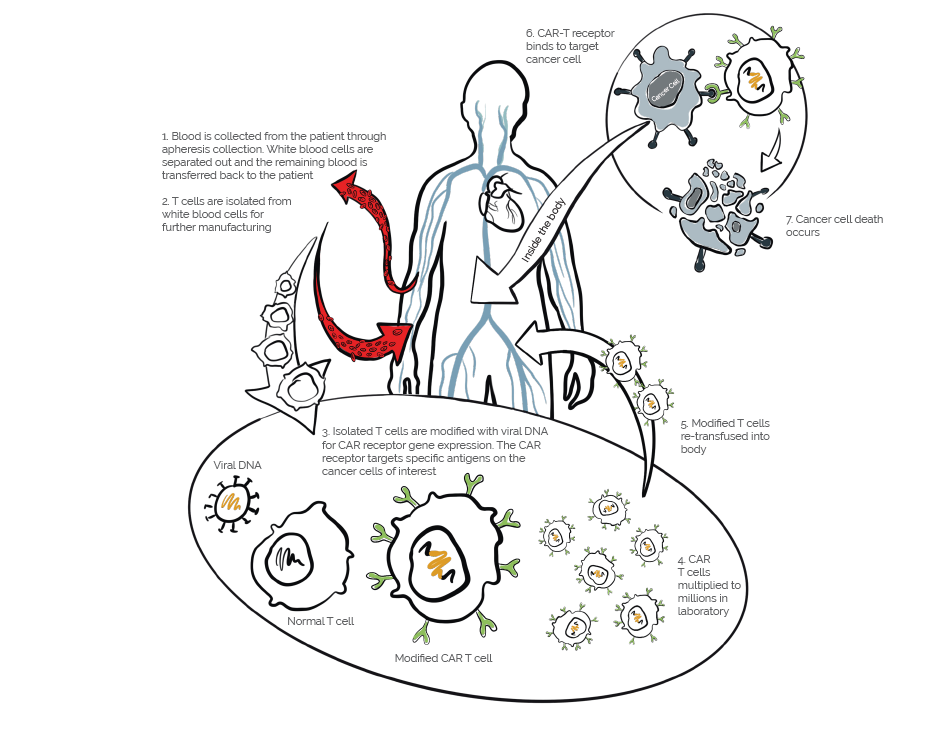
Autologous chimeric antigen receptor (CAR) T cell therapy is breakthrough treatment for certain types of blood cancers. The treatment involves isolating a person’s own immune cells and manufacturing them to recognize and kill target antigens on the cancer cells of interest. A patient’s T cells are modified with the CAR gene, which then causes the T cell to express the chimeric antigen receptor. These engineered CAR-T cells are then multiplied in the lab by the millions and reinfused back into the patient’s bloodstream. The T cells then locate and kill cancer cells with the target antigen, and when successful, offer lasting remission rates that are higher than existing cancer treatments.
CGT Healthcare - The Missing Link
With CAR-T therapy, time is of the essence. As more therapies move to approval stages, the need for a streamlined collection, manufacturing and re-transfusion process is imminent. One strategy to speed up CAR-T timelines is to develop a more efficient manufacturing process that can produce large quantities of CAR-T Cells in a shorter time frame. Additionally, researchers are investigating ways to streamline the regulatory approval process, which can cause significant bottlenecks. With these strategies in place, researchers hope to accelerate this process, allowing more patients to benefit from this promising new treatment option.
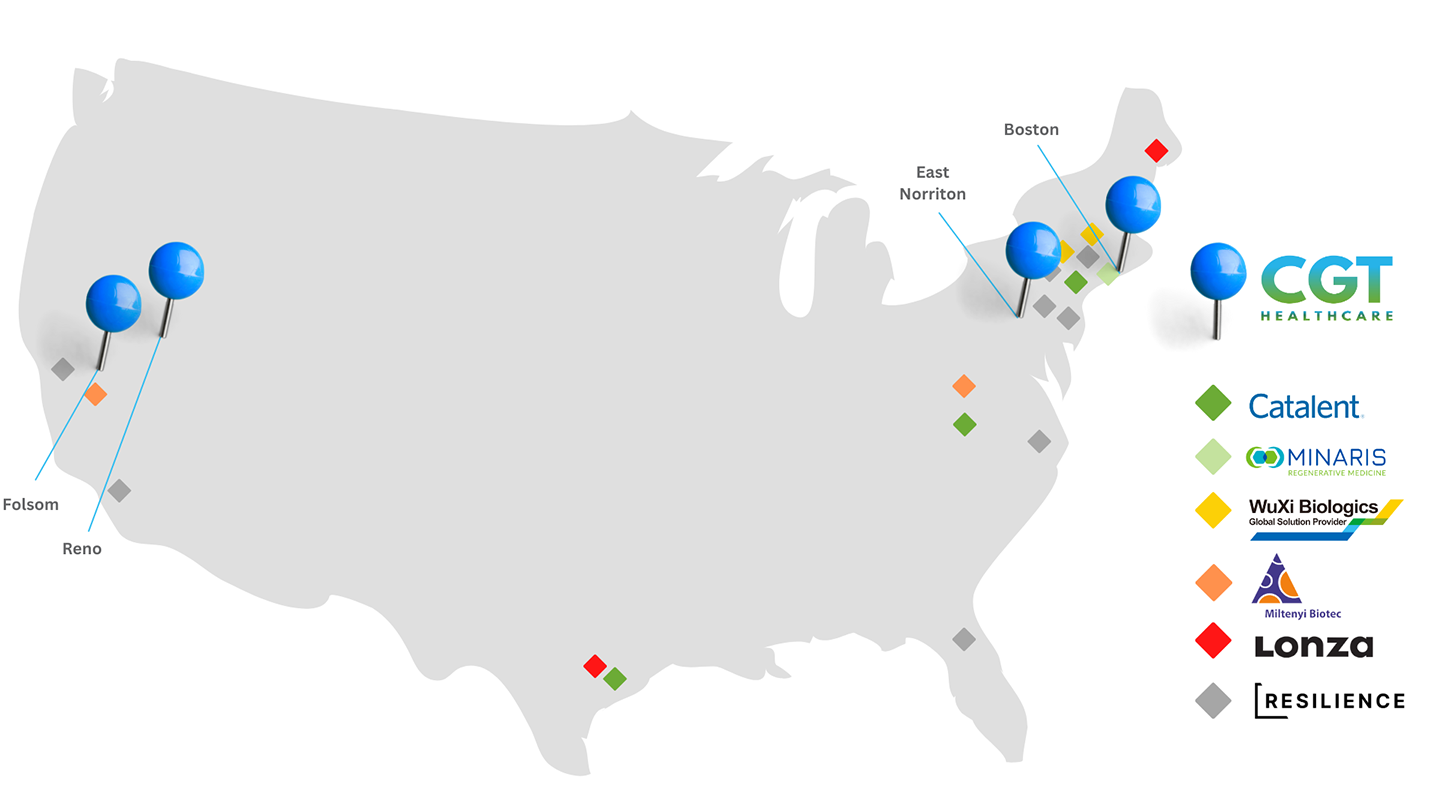
In an effort to accelerate the apheresis and manufacturing steps, CGT Healthcare offers patient intake for apheresis collections at 6 collection centers across the nation - providing clinical grade Leukopaks ready for CAR-T manufacturing and re-infusion. We can house and operate cell manufacturing equipment under the hospital’s regulatory compliance to alleviate the need for cumbersome laboratory equipment in confined hospital settings. CGT Healthcare is strategically located in close proximity to major healthcare and biotech spheres to allow for greater patient access, and rapid turnaround times from point of collection to re-infusion eliminating the costly logistics in between.
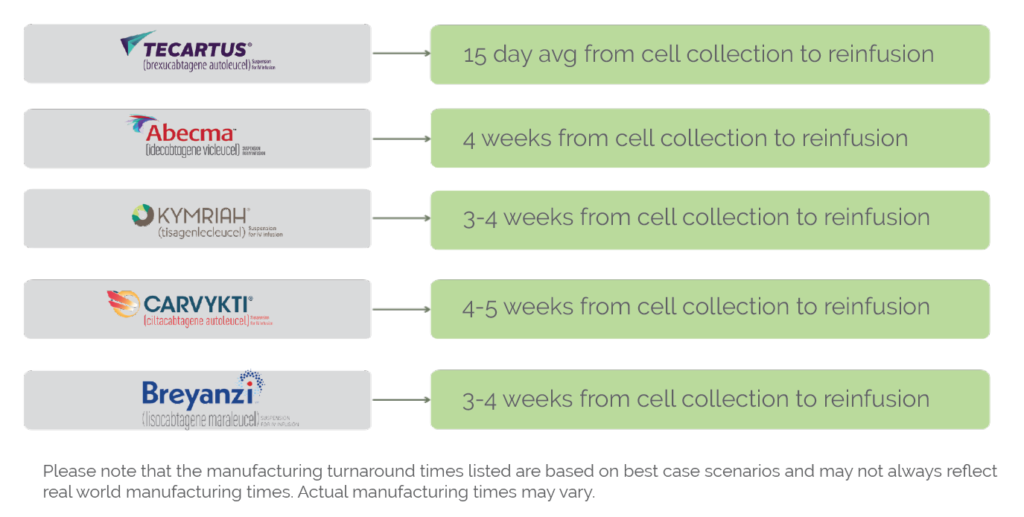
The manufacturing process for FDA-approved CAR-T therapies is a complex and time-consuming process that typically takes several weeks to complete. The exact timeline for CAR-T manufacturing can vary depending on a variety of factors, including the patient’s individual needs and the specific manufacturing protocols used by the therapy’s manufacturer, however the average time frame is currently 24 days. Below are the specific timetables for each FDA approved CAR-T manufacturer. CGT Healthcare hopes to eventually accelerate these timelines by days if not weeks.
Strategic Locations for Manufacturing Partners
CGT Healthcare has created a strategic network of cell collection centers with the cell and gene therapy industry in mind. Knowing that many therapeutic developers utilize third party manufacturing or CDMOs, we fulfill clinical collections intended for further manufacturing from all 6 collection facilities near major US-based CDMO partners. We can also work directly with your CDMO or manufacturing partner to simplify patient collections. Utilizing our coast-to-coast footprint, CGT Healthcare can mitigate risks caused by natural disasters, COVID closures, and other unforeseen circumstances.
Learn More
If you're interested in learning more about how CGT Healthcare can help you bring therapies to patients faster, please don't hesitate to contact us at the email address or phone number listed on our website. Our expert team is always happy to answer any questions you may have and help you explore CGT Healthcare's offerings.
If you're interested in learning more about CAR-T therapy as it relates to the hospital, we invite you to request our brochure by filling out our contact form and requesting a copy! Our brochure provides a comprehensive overview of CAR-T therapy, including its benefits, risks, and potential applications.
