GMP Leukopaks Engineered for Clinical Excellence
HIGH-YIELD, HIGH-QUALITY STARTING MATERIALS TRUSTED IN CELL AND GENE THERAPY TRIALS AND MANUFACTURING.
GMP leukopaks for critical applications in cell and gene therapy, collected, processed, and delivered with unmatched precision and regulatory assurance.
Meet Your Clinical Team






Why Choose CGT Global’s GMP Leukopaks?
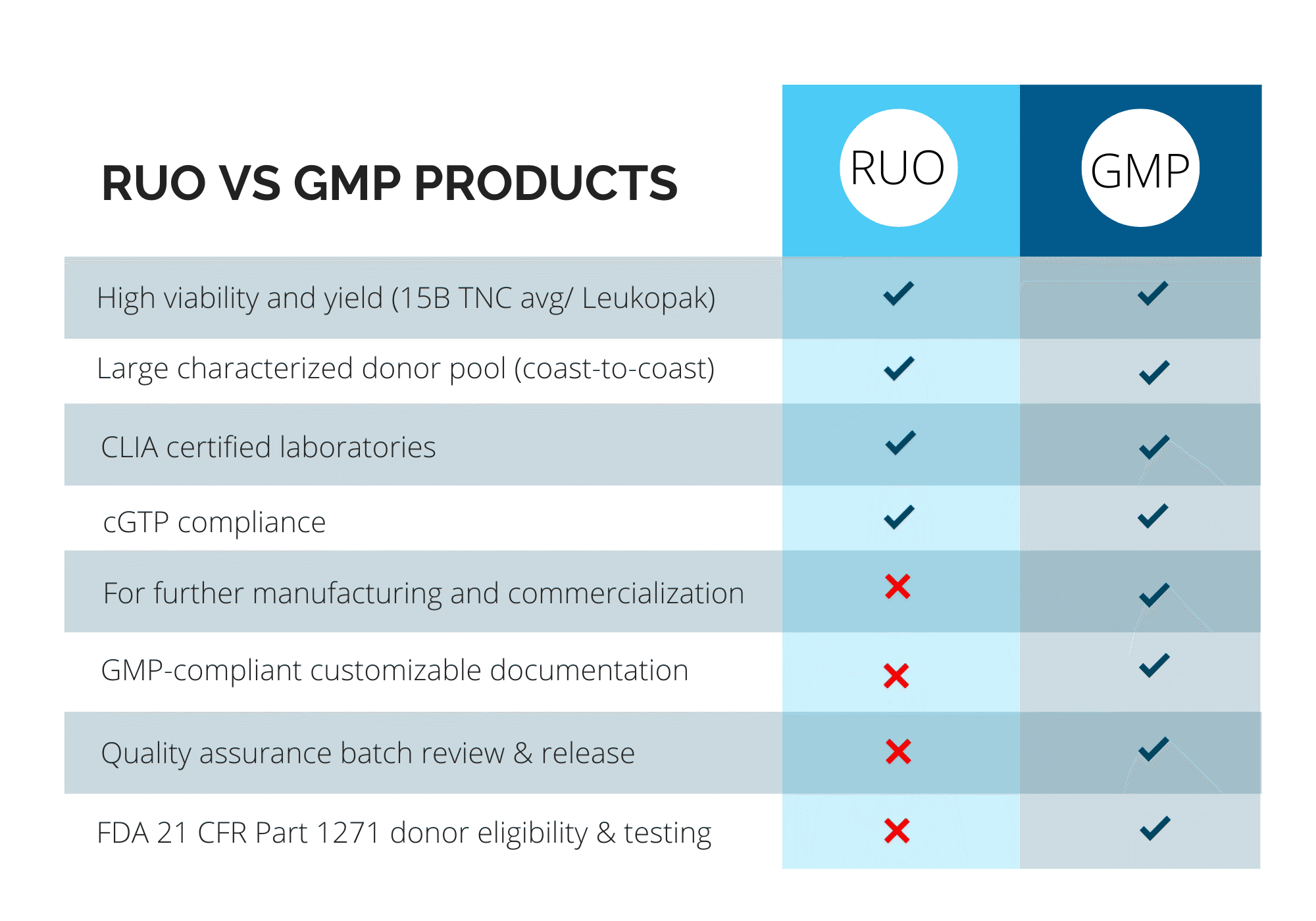
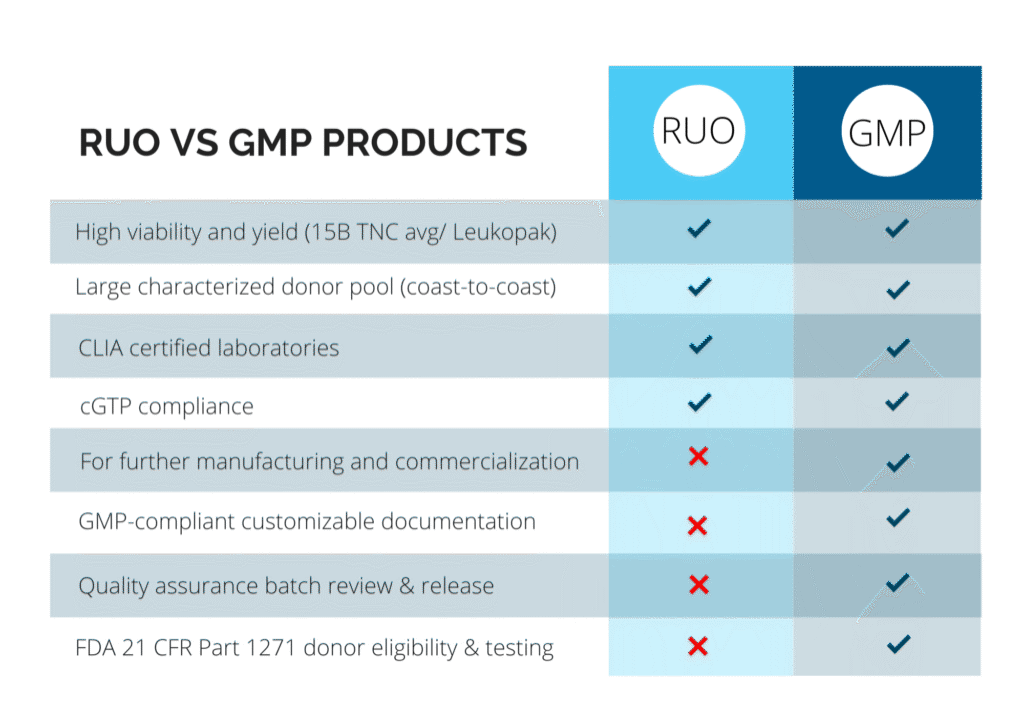
Pre-Clinical (RUO) vs. Clinical (GMP) Products
At CGT Global, we understand that every project is different. That’s why we work closely with each client to determine the specific compliance requirements based on how our materials will be used.
With over a decade of experience as a trusted provider of RUO and GMP-compliant healthy donor materials, we offer flexible, customizable solutions to support researchers and drug developers at every stage of their work.
Partner with CGT Global, where expertise meets flexibility.
Dependable Donors for Reproducible Science
At CGT Global, we recognize that allogeneic therapies, such as CAR-T, demand the right donor, qualified through rigorous screening and compliant with 21 CFR 1271 Subpart C. These donors must meet strict eligibility requirements and undergo extensive testing before contributing raw material for clinical manufacturing.
To address this critical need, we offer a unique, program-specific donor solution managed by our specialized donor recruitment and collection team. Whether you’re launching a new program or scaling an existing one, our team works closely with you to build a tailored strategy that supports every stage, from donor recruitment through to product fulfillment.
Start Your Program with Confidence
CGT Global will guide you through the startup and ordering process, collaborating with you to deliver a solution that fits your exact needs. Contact us today to schedule an introductory or exploratory evaluation of your program
- Location-specific donor collection in geographic proximity to your Manufacturing facility
- Complex donor screening and pre-qualification
- Specific donor recruitment or eligibility criteria
- Additional infectious disease testing (HHV6, 7, 8, Babesia, Toxoplasma, etc.)
- IRB-approved donor Informed Consent Forms (ICF)
- Supplemental Donor History Questionnaires (DHQ)
- Customized Certificate of Analysis (CoA)
- Analytical testing & other donor or collection characterization
- Shipping containers and carriers
This service includes: Leukapheresis and Whole Blood
- FDA compliant Infectious Disease Testing panels
- Additional Infectious Disease Testing provided by approved vendors
- Donor characterization including HLA, KIR, genomic screening
- Location-based donor recruitment for supply chain optimization
- Dedicated donor management to your clinical program
- Further customization by evaluation
FAQ's
-
- Clinical-Grade Materials On Demand
GMP Leukopaks and whole blood from FDA-registered, CLIA-certified state of the art laboratories, ready for regulatory pathways and high-quality clinical trial consistency. - Speed & Reliability with Coordinated Logistics
Rapid, same-day collection and shipment at the major Biotech hubs in the Massachusetts, Philadelphia and California. — and worldwide shipments with our trusted logistics partners. Through our trusted logistics partners, wWe ensure reliable global delivery of fresh and cryopreserved materials with validated cold-chain systems. Our team proactively manages documentation and compliance to prevent customs delays, ensuring your time-sensitive materials move seamlessly across borders and arrive on schedule. - Advanced Cell Processing for Trial-Ready Materials
Automated isolations via CliniMACS Prodigy® and utilizing BioSpherix Xvivo X2®— cells custom-prepared to match your protocol. - Regulatory & Documentation Support
Full traceability, IND-ready documentation, IRB support, and donor recall options to streamline compliance and reduce risk. - Scalable Partnership for Every Trial Phase
From preclinical to pivotal trials, we flex with your needs, supporting custom donor projects, specialty isolations, and multi-site studies. - Expert Collaboration, Not Just a Vendor
Dedicated clinical team, responsive service, and over 15 years of experience guiding cell and gene therapy innovators to commercialization.
- Clinical-Grade Materials On Demand
Good Manufacturing Practice (GMP) refers to a set of regulations ensuring that products are consistently produced and controlled according to high-quality standards. It guarantees that the starting materials for clinical trials are safe, effective, and reproducible.
CGT Global Clinical Leukopaks are collected from IRB-approved consented donors or and patients at coast-to-coast FDA registered collection centers by qualified and trained staff using the Terumo Spectra Optia® cMNC collection protocol. Donor eligibility is in compliance with FDA 21 CFR subpart C.
We offer both fresh and cryopreserved GMP Leukopaks.
GMP Leukopak is shipped immediately after collection for same-day or next day delivery through our trusted logistics vendors censuring fast and reliable delivery for your projects.
Yes, we provide highly customizable cell isolations from our GMP Leukopaks. Whether you need specific immune cell types or require a tailored process, our team is here to support your needs.
Our GMP Leukopaks and Whole Blood are collected under strict sterile protocols. GMP Cryopreserved Leukopaks are tested in accordance with state and Federal requirements for sterility and other pathogen testing.
Tour Our GMP Cell Processing Facility
Step inside CGT Global’s advanced GMP laboratory, where precision meets compliance. Our facility is purpose-built to support clinical-grade manufacturing, providing a controlled environment for the processing, expansion, and cryopreservation of critical cell populations.
Cryogenic Storage & Preservation
CGT Global GMP Laboratory houses LN2 cryogenic storage systems, including vapor-phase cryo tanks equipped with automated temperature monitoring and backup power redundancy to maintain sample integrity at all times. All cryopreserved materials undergo validated controlled rate freezing protocols and are tracked through our validated inventory system.
Closed-System Cell Processing with CliniMACS Prodigy®
We utilize automated, closed-system cell isolation and enrichment, minimizing contamination risk and supporting GMP-grade reproducibility. For phenotypic analysis of expanded cell populations, we employ flow cytometry. Together, these technologies support applications such as CD34+ enrichment, T-cell subset selection, and detailed immunophenotyping.
Integrated QC & Traceability
Samples move through a fully integrated workflow that includes:
- Real-time viability and phenotyping via flow cytometry
- Mycoplasma and endotoxin testing for Cryopreserved GMP Leuakopaks
- Digital chain-of-custody tracking
We welcome collaborators and clients to tour the facility virtually or in person, and witness firsthand the rigor and reliability that define CGT Global’s clinical operations.
.
Clinical Leukopaks and Whole Blood Products
-
GMP Fresh Clinical Leukopaks
Select options This product has multiple variants. The options may be chosen on the product page -
GMP Cryopreserved Clinical Leukopaks
Select options This product has multiple variants. The options may be chosen on the product page -
GMP Clinical Whole Blood
Select options This product has multiple variants. The options may be chosen on the product page