Estimated reading time: 7 minutes
From RUO to cGMP, CGT Global delivers quality, consistency, and regulatory readiness to help researchers advance therapies from discovery to patients.
Executive Summary
CGT Global accelerates cell and gene therapy development from discovery to commercialization. By providing rapid and reliable access to high-quality RUO and cGMP-certified materials and operating FDA- and CLIA-certified clinics co-located with cell specific labs, CGT Global ensures speed, consistency, and regulatory-ready CMC packages. This integration helps sponsors avoid delays, streamline clinical trials, and expand patient access to life-changing therapies.
Advancing cell and gene therapies can feel like climbing Mount Everest. The path is steep, the risks are high, and only the most prepared reach the summit.
CGT Global is on a mission to transform the life science and medical industries by accelerating this journey from discovering new treatments to delivering them to patients.
The environment may be unpredictable and the regulatory climate ever-changing, but innovators can anchor themselves in what matters most: the quality of their science and the quality of their product.
Today, we explore how CGT Global expedites research, accelerates clinical trials, and streamlines the path to commercialization to broaden patient access to life-changing therapies.
Expediting Research
The importance of securing starting materials on time is critical for preclinical researchers who set out to blaze the trail of discovering new therapies. Even small delays can disrupt experimental timelines. A reliable supply chain of high-quality biological material, delivered with rapid turnaround, is essential to keeping discovery milestones on track.
CGT Global delivers Leukopaks, isolated primary cells (CD34+ HSPC, NK cells, PBMCs, and others) bone marrow, whole peripheral and cord blood to scientists with speed and precision. Below are the average lead times for biospecimen delivery.
Average Biospecimen Request-to-Delivery Times*
- Whole blood: 3 days
- Leukopaks: 4-5 days
- Bone marrow: 5-7 days
*Actual lead times may vary.
Research Use Only (RUO) biological materials are essential for proof-of-concept validation, characterizing the mode of action, and early toxicology testing. Proving the science is solid with a deep understanding of the therapeutic mechanism of action is the foundation of creating safe and effective next-generation therapies. By ensuring researchers have timely access to healthy and disease-state donor material, CGT Global helps shave months to years off experimental timelines.
A recent case demonstrates our dependability: 600 vials were transported from the West Coast to the East Coast in under 72 hours, supported by a contingency plan that deployed two aircraft to mitigate risk. Additionally, last month alone, over 370 orders left our facility without a single shipping delay.
CGT Global provides both RUO and current Good Manufacturing Practice (cGMP) blood and cell products to support every step of the research and development journey, empowering scientists to move discoveries from hypothesis to healing.
Accelerating Clinical Trials
Without reliably sterile, viable, current Good Manufacturing Practice (cGMP) certified biological starting materials, translating next-generation immunotherapies from scientific insights into patient care will stall before it begins.
CGT Global’s Clinical Solutions starts with expert donor management from donor recruitment to donor screening. With a well-established pool of clinical donors, CGT Global provides cGMP cells isolated using the CliniMACS Prodigy® and cGMP Leukopaks from leukapheresis using the Terumo Spectra Optia®.
These cGMP human samples support the critical steps of moving a candidate therapy from generating data at the bench to first-in-human dosing. Across this continuum of development, cGMP products are essential for training runs, process engineering, IND-enabling studies, early- and late-phase clinical trials, commercial manufacturing, and long-term post-marketing surveillance.
Chemistry, Manufacturing, and Controls (CMC) are subject to heavy scrutiny by the FDA. Nearly three-quarters of FDA rejections are not due to the underlying science, but to shortcomings in logistics, manufacturing, and process coordination. Without a bulletproof CMC strategy, programs risk clinical holds that delay timelines and increase overall development costs.
By providing dependable cGMP-compliant starting materials and time-tested donor , CGT Global helps ensure that logistics and supply are never the reason a promising therapy fails to reach the market.
Streamline Commercialization
Millions of people today could benefit from cell and gene therapies, yet obstacles such as cost and current healthcare infrastructure still stand in the way. Today, many hospitals lack the facilities, equipment, and trained personnel to efficiently and effectively deliver cell and gene therapies at scale.
CGT Global closes this critical gap between collection and treatment by facilitating the delivery of cell and gene therapies like autologous CAR-T to patients. For example, CGT Healthcare provides patient intake for apheresis collections at clinics located across the US. Clinical-grade Leukopaks are collected on site, ready for CAR-T manufacturing and reinfusion.
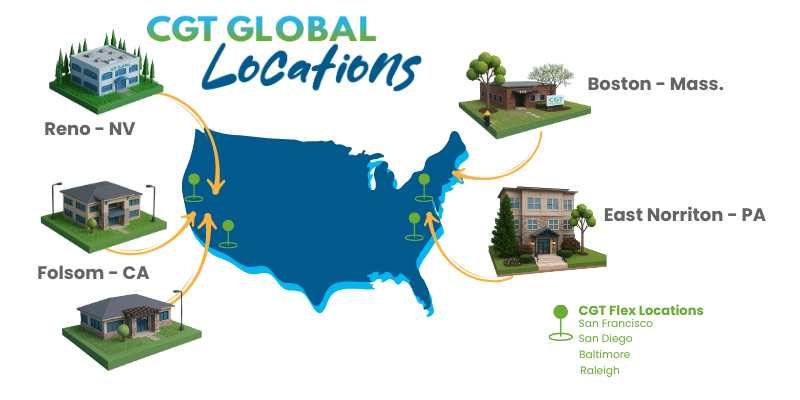
CGT Global’s three clinics are located near major healthcare and biotech hubs, enabling greater patient access and rapid turnaround from collection to use, while eliminating costly logistical delays. In addition, CGT Healthcare can operate cell manufacturing equipment in one of two BioSpherix Xvivo X2 closed system ISO5 GMP units under hospital SOPs and regulatory oversight, streamlining cell therapy operations and reducing the need for hospitals to maintain complex laboratory infrastructure.
Historically, cell and gene therapy has only been available to a limited number of patients, but this is rapidly changing in 2025. The regulatory landscape is shifting as the FDA eliminated Risk Evaluation and Mitigation Strategies (REMS) requirements to lower barriers to CAR-T delivery, while government-sponsored healthcare launched outcomes-based reimbursement models such as the CGT Access framework to expand access to Casgevy, the sickle cell gene therapy. These ecosystem level changes underscore the urgent need for proper infrastructure and tested logistics to deliver life-changing treatments to patients safely and effectively.
By bridging the “last mile” from patient to therapy, CGT Global helps accelerate the delivery of groundbreaking treatments like CAR-T, which can be administered as a single infusion rather than multiple rounds of chemotherapy. This shift not only shortens treatment timelines but also redefines what is possible for patients.
Partnering for the Future
Bringing new cell and gene therapies to life is a challenging undertaking, but the reward is profound: medical interventions designed to precisely address the root cause of disease. Success on this journey requires trusted partners who can ensure quality at every stage.
That’s why working with a single vendor from research use only RUO through cGMP manufacturing is so important. Consistency streamlines operations, reduces variability, and strengthens the CMC package that the FDA relies on to determine whether a therapy can be consistently and safely produced.
At CGT Global, quality begins with pre-qualified vendors, equipment, and consumables, and it carries through to every outgoing shipment. By choosing CGT Global, you gain more than a supplier – you gain a partner dedicated to helping you deliver safe, reliable, and transformative therapies.
Accelerating Your Cell & Gene Therapy Program
Are you ready to partner with collaborators who understand the high stakes and complex demands of cell and gene therapy development?
Contact our team to discover how CGT Global’s, Research, Clinical, and Healthcare Solutions can help bring your next treatment to patients faster.
Since 2010, CGT Global has been supporting scientists leading medical innovation with donor management, human biospecimen procurement, and primary cell isolations. Our three FDA and CLIA-certified Stem Cell Collection Centers located in the US (Folsom, CA; East Norriton, PA; and Boston, MA) include collection clinics co-located with cell processing laboratories, ensuring quality and high cell viability through rapid sample processing. Our streamlined recruitment and screening protocols have established donor pools with over 6,000 donors on the East and West Coast with over 13,000 donors total. Our large donor pool is diverse, recallable, and well characterized, giving you streamlined access to the high-quality Leukopaks and primary cells essential for bringing your novel biologics-based therapeutics to market faster. Equipped with RUO and GMP capabilities, we look forward to supporting your translational research along the therapeutic commercialization lifecycle, spanning development to post-approval scale-up. As scientists helping scientists, we pride ourselves on going above and beyond to meet the needs of our research collaborators with many customization options.
Want to learn more? Reach out to talk to our team to learn how partnering with CGT Global’s Clinical Solutions will accelerate the development of your next life-changing therapy.