Bone Marrow CD34+ Stem/Progenitor Cells, Fresh
CD34 is a glycosylated transmembrane protein and represents a well-established marker for human hematopoietic stem and progenitor cells in peripheral blood, bone marrow, and cord blood. CD34+ cells are self-renewing, multipotent stem cells that give rise to all blood cells of the immune system through a process called hematopoiesis.
Since our founding in 2010, CGT Global has pursued our mission to transform healthcare as we accelerate cell and gene therapy research and clinical trials, streamline the commercialization of new treatments, and map the last mile to patient access to these life-changing remedies. By innovating each stage in the cycle; development, commercialization, and delivery, we reduce the overall cost of the care and multiply access points so that millions can receive cutting edge, life-saving gene and cell therapies.

Description
CD34 is a glycosylated transmembrane protein and represents a well-established marker for human hematopoietic stem and progenitor cells in peripheral blood, bone marrow, and cord blood. CD34+ cells are self-renewing, multipotent stem cells that give rise to all blood cells of the immune system through a process called hematopoiesis. As hematopoietic stem cells progress through hematopoiesis they generate the myeloid (monocytes, macrophages, granulocytes, megakaryocytes, dendritic cells, erythrocytes) and lymphoid (T cells, B cells, NK cells) lineages. The highly specialized cells that arise from hematopoietic stem cells work collaboratively in defending the body against infection and disease.
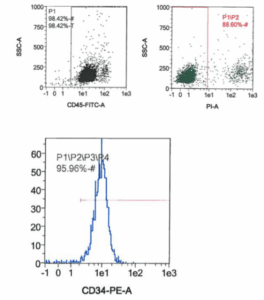
Bone marrow mononuclear cells (MNCs) are separated from whole bone marrow by a density gradient centrifugation protocol.CD34+ cells are positively selected using immunomagnetic anti-CD34 (clone QBEND10) microbeads from bone marrow mononuclear cells. Isolated primary cells are characterized by flow cytometry to ensure a highly pure and viable cell population.
Cells were obtained using Institutional Review Board (IRB) approved consent forms and protocols.
Additional information
| Anticoagulant | |
|---|---|
| Format | |
| Grade | |
| Species | |
| Cell and Tissue Source | |
| Disease State | |
| Donor Attributes |
Product Information Sheet
Material Safety Data Sheet
Publications
- Eksioglu et al. (2017) Novel Therapeutic Approach to Improve Hematopoiesis in Low Risk MDS by Targeting MDSCs with the Fc-engineered CD33 Antibody BI 836858. Leukemia 1-9. doi:10.1038/leu.2017.21. Abstract
- Hudak et al. (2014) Glycocalyx Engineering Reveals a Siglec-Based Mechanism for NK Cell Immunoevasion. Nat Chem Biol 10: 69-75. doi:10.1038/nchembio.1388. Abstract
- Modarai et al. (2018) Efficient Delivery and Nuclear Uptake Is Not Sufficient to Detect Gene Editing in CD34+ Cells Directed by a Ribonucleoprotein Complex. Mol Ther Nucl Acids 11: 116-129. doi.org/10.1016/j.omtn.2018.01.013. Article
- Cheng et al. (2019) S100A9-Induced Overexpression of PD-1/PD-L1 Contributes to Ineffective Hematopoiesis in Myelodysplastic Syndromes. Leukemia. doi.org/10.1038/s41375-019-0397-9. Article
- Lo et al. (2020) Potent In Vitro Activity of β-D-4ʹ-Chloromethyl-2ʹ-Deoxy-2ʹ-Fluorocytidine Against Nipah Virus. Antivir Res doi.org/10.1016/j.antiviral.2020.104712. Abstract
- Modarai et al. (2020) Precise and Error-Prone CRISPR-Directed Gene Editing Activity in Human CD34+ Cells Varies Widely Among Patient Samples. Gene Therapy. https://doi.org/10.1038/s41434-020-00192-z. Article