Fresh Mobilized Leukopaks | PLX
Human mobilized peripheral blood Leukopaks provide a rich source of hematopoietic stem cells as compared to traditional Leukopaks. This allows researchers greater reproducibility of experiments from a single donor (minimize donor-to-donor variation), increase in scalability of experiments from a single product, and a decrease in same cell variability by obtaining high yields of specific cell types.
- Custom injection regiments with Plerixafor leading to billions of cells per collection
- CD34+ and 7 panel emailed on the day of collection to allow researchers to set up reagents in their lab
- Weekend collections available
- Isolation of CD34+ to save time on isolations and consumables
Since our founding in 2010, CGT Global has pursued our mission to transform healthcare as we accelerate cell and gene therapy research and clinical trials, streamline the commercialization of new treatments, and map the last mile to patient access to these life-changing remedies. By innovating each stage in the cycle; development, commercialization, and delivery, we reduce the overall cost of the care and multiply access points so that millions can receive cutting edge, life-saving gene and cell therapies.

Description
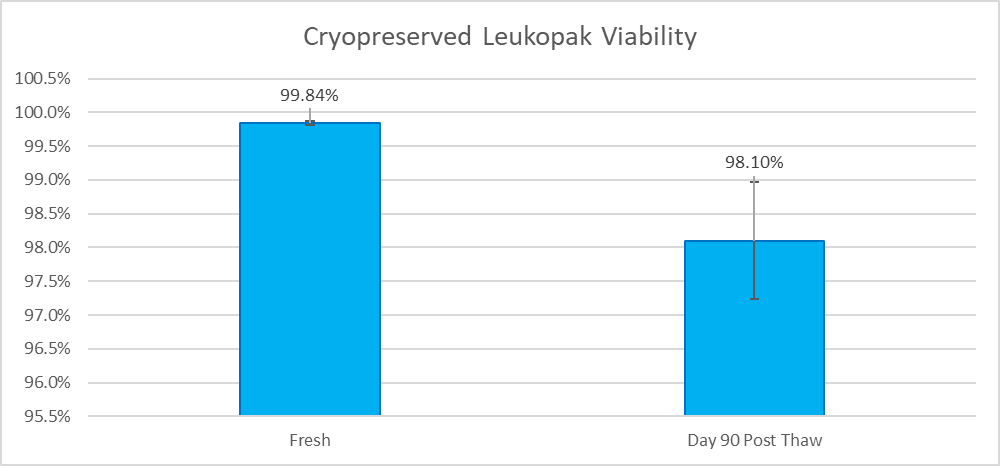
Mobilized Leukopaks are collected from healthy IRB consented donors that are injected with 240µg/kg of Plerixafor the day before collection, which stimulates the bone marrow to produce hematopoietic stem cells and mobilizes them into the bloodstream. Mobilized mononuclear cells are collected by a trained technician using the Spectra Optia® Apheresis System. After collection, mobilized Leukopaks are checked by flow analysis to verify the percentage of granulocytes, mononuclear cells, CD34+ cells, and cell viability.
• 2x10e10 TNCs/1 bag
• Enriched PBMCs
• Enriched CD34+ cells
• Low hematocrit
• Low granulocytes
Cells were obtained using Institutional Review Board (IRB) approved consent forms and protocols.
Custom Orders
We work closely with our customers to provide them with the product they need. For a custom order, please call and speak to one of our sales specialists at 530-303-3828.
Additional information
| Anticoagulant | |
|---|---|
| Format | |
| Grade | |
| Species | |
| Cell and Tissue Source | |
| Disease State | |
| Donor Attributes |