Coming Soon



Why Choose CGT Global Mobilized Leukopaks?
Extensive, Well-Characterized Mobilized Donor Network
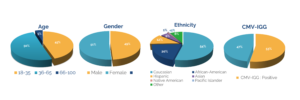
Access G-CSF and PLX-mobilized donors screened under IRB-approved protocols. Each donor profile includes deep demographic, phenotypic, and HLA data, along with mobilization regimen details and cell yield history.
Whether you’re scaling CAR T, NK, or stem cell programs, our mobilized donor pool ensures you receive high-yield, reproducible starting material with complete transparency.
Donor Recallability
When reproducibility is essential, we make it possible to re-collect mobilized material from the same donor.
This allows your team to perform batch-to-batch validation, assess mobilization response consistency, and standardize your production workflow — minimizing biological variability in translational studies.
Three FDA-Registered, CLIA-Certified Facilities
Our bi-coastal collection network supports mobilized leukapheresis at FDA-registered, CLIA-certified sites near major biotech and cell therapy hubs.
This infrastructure enables rapid scheduling, shorter donor prep times, and localized fulfillment for time-sensitive mobilized collections.
Same-Day Collection & Shipping
Mobilized leukopaks are collected and shipped the same day, preserving peak viability and CD34+ integrity.
Our logistics protocols maintain temperature control and chain-of-custody compliance from donor to lab, ensuring your mobilized starting material arrives fresh, verified, and ready for isolation.
Integrated Supply Chain
CGT Global oversees every stage of mobilized collection — from donor recruitment through G-CSF/PLX administration to final delivery.
By eliminating third-party dependencies, we maintain full visibility and quality control, shorten lead times, and remove uncertainty from the supply chain.
Dedicated Client Service Associate Support
Each mobilized project is managed by a dedicated Client Scientific Associate (CSA) who coordinates donor scheduling, documentation, and shipping.
Your CSA provides real-time updates throughout mobilization, collection, and shipment, ensuring your material is delivered on time, compliant, and exactly as specified.
Why Choose CGT Global Leukopaks?
One of the Largest, Most Characterized Mobilized Donor Networks
Access G-CSF and PLX-mobilized donors screened under IRB-approved protocols. Each donor profile includes deep demographic, phenotypic, and HLA data, along with mobilization regimen details and cell yield history.
Whether you’re scaling CAR T, NK, or stem cell programs, our mobilized donor pool ensures you receive high-yield, reproducible starting material with complete transparency.
Donor Recallability
When reproducibility is essential, we make it possible to re-collect mobilized material from the same donor.
This allows your team to perform batch-to-batch validation, assess mobilization response consistency, and standardize your production workflow — minimizing biological variability in translational studies.
Same-Day Collection & Shipping
Mobilized leukopaks are collected and shipped the same day, preserving peak viability and CD34+ integrity.
Our logistics protocols maintain temperature control and chain-of-custody compliance from donor to lab, ensuring your mobilized starting material arrives fresh, verified, and ready for isolation.
Three FDA-Registered, CLIA-Certified Facilities
Our bi-coastal collection network supports mobilized leukapheresis at FDA-registered, CLIA-certified sites near major biotech and cell therapy hubs.
This infrastructure enables rapid scheduling, shorter donor prep times, and localized fulfillment for time-sensitive mobilized collections.
Integrated Supply Chain
CGT Global oversees every stage of mobilized collection — from donor recruitment through G-CSF/PLX administration to final delivery.
By eliminating third-party dependencies, we maintain full visibility and quality control, shorten lead times, and remove uncertainty from the supply chain.
Dedicated Client Service Associate Support
Each mobilized project is managed by a dedicated Client Scientific Associate (CSA) who coordinates donor scheduling, documentation, and shipping.
Your CSA provides real-time updates throughout mobilization, collection, and shipment, ensuring your material is delivered on time, compliant, and exactly as specified.
Testimonials
"The CGT Global CSA team is outstanding, they know our project inside and out and proactively handle every detail. From our first inquiry to delivery, they made the process seamless. It’s rare to work with a team that combines scientific expertise with genuine human support."
— Dr. Karen M., Immunotherapy Research Scientist
"We rely on CGT Global leukopaks because the quality is always consistent. Every batch meets our strict experimental standards, and the viability and cell counts are exactly as promised. That level of reproducibility is critical for our preclinical work."
— Dr. Alex P., Senior Cell Therapy Researcher
"CGT Global delivers on time, every time. Same-day collections and next-day shipments mean our experiments never have to wait. Their attention to logistics and scheduling makes them a true partner in our research."
— Dr. Priya S., Biotech Project Manager
"We’ve been sourcing leukopaks from CGT Global for several years. Their facilities, donor pool, and scientific support are unmatched. But what really sets them apart is the team — they understand both the science and the practical needs of researchers, making every order effortless."
— Dr. Michael L., Translational Medicine Lead

Our Facilities
Our CLIA-certified clinical processing lab delivers FDA clinical-grade leukopaks with uncompromising quality — backed by validated protocols, advanced cryopreservation, mobilization, and custom cell enrichment.
Feeding into the lab are CGT Global’s FDA-registered collection clinics in Boston, Philadelphia, and Folsom, equipped with Spectra Optia® systems and staffed by expert nurses and scientists. Supporting these sites is our Reno facility, home to 50,000 sq. ft. of state-of-the-art cryogenic storage capacity. With strategic locations, we offer same-day delivery to major biotech hubs and next-day delivery nationwide, sourcing from a diverse, recallable donor pool to meet your exact study needs.
Boston, MA – Advanced Collection Hub in the Heart of Biotech
Located minutes from the world’s leading research institutions, our Boston facility is FDA-registered and CLIA-certified, equipped with state-of-the-art apheresis technology, and staffed by experienced nurses and scientists. Its proximity to the Greater Boston biotech corridor enables same-day delivery to local labs and rapid turnaround for East Coast clients.
Philadelphia, PA – Scalable Clinical & Research Collections
Our Philadelphia site serves as a high-capacity hub for both GMP and research-grade collections. FDA-registered and CLIA-certified, this location is optimized for large-scale donor programs, mobilized collections, and complex custom processing. Centrally positioned, it offers next-day delivery to most U.S. destinations and efficient global shipping connections.
Folsom, CA – West Coast Speed with National Reach
Strategically located to serve West Coast biotech hubs, our Folsom facility delivers fresh collections to California-based researchers the same day, with rapid shipping across the Pacific and to the rest of the U.S. Fully FDA-registered and CLIA-certified, it offers the same stringent quality standards and customizable donor options as our East Coast sites.
Reno, NV – Cryo-Facilities in Strategic Locations
Located in a major transportation and logistics hub, our Reno headquarters offers a 50,000 sq ft freezer with a -80 degree space.




Deep Donor Characterization
Our donor database includes:
-
Demographics and medical history
-
Disease characterization and outcome data
-
CBC reports, vaccination status, and viral-negative screening
-
Immunophenotyping via flow cytometry
-
HLA-typing and recallable donors
All donors are initially screened before their first donation and rescreened every 3 months to maintain our strict standards.
Custom Processing & Assays
In addition to leukopak collection, our in-house labs can provide:
-
PBMC isolation
-
BMMCs, DTCs, enriched cell populations
-
T-cell expansion
-
Immunophenotyping panels
-
Purity assessments
-
ELISA, ELISpot, Fluorospot assays
-
Custom assay development
Select donors by demographic or disease state, specify processing and isolation methods, and request packaging that fits your lab workflow. Every leukopak is made to match your study design, not the other way around.