Fresh Leukopaks
Fresh Leukopaks
CGT Global Leukopaks are and collected from IRB-approved consented healthy donors at FDA registered collection clinics by qualified staff and shipped same day.
- Fresh Leukopaks Delivered in within 24 hours after collection
- Leukopak collections scheduled as soon as 48 hours from quote request
The CGT Global Difference
CGT Global is a Biospecimen Provider for a wide range of clinical and preclinical research projects. Our state-of-the-art laboratories and collection centers are located nationwide and provide end-to-end services: These services include:
- Process, isolate, and cryopreserve cells immediately after donor collection
- Amazing customer service and fast shipping
- Customize products to distinct study specifications
- Retain specific donor demographics and a safe, highly recallable donor pool
- HLA Typed Primary Cells
- Biobanked cells

Description
Our Fresh Leukopaks are collected from healthy IRB consented donors, that have been tested negative for HBV, HCV, and HIV. Leukopaks are collected using the Spectra Optia® Apheresis System, where cell-rich mononuclear cells are extracted using a continuous flow system directly into a sterile collection bag containing the anticoagulant ACD-A. CGT Clinics are CLIA Certified and FDA Registered. Up to 12 Fresh Leukopak collections can be performed on the same donor in a given year, providing an ample amount of PBMCs for large-scale experiments and research studies. Additionally, 200-300mL of plasma can be collected at the time of the donor draw.
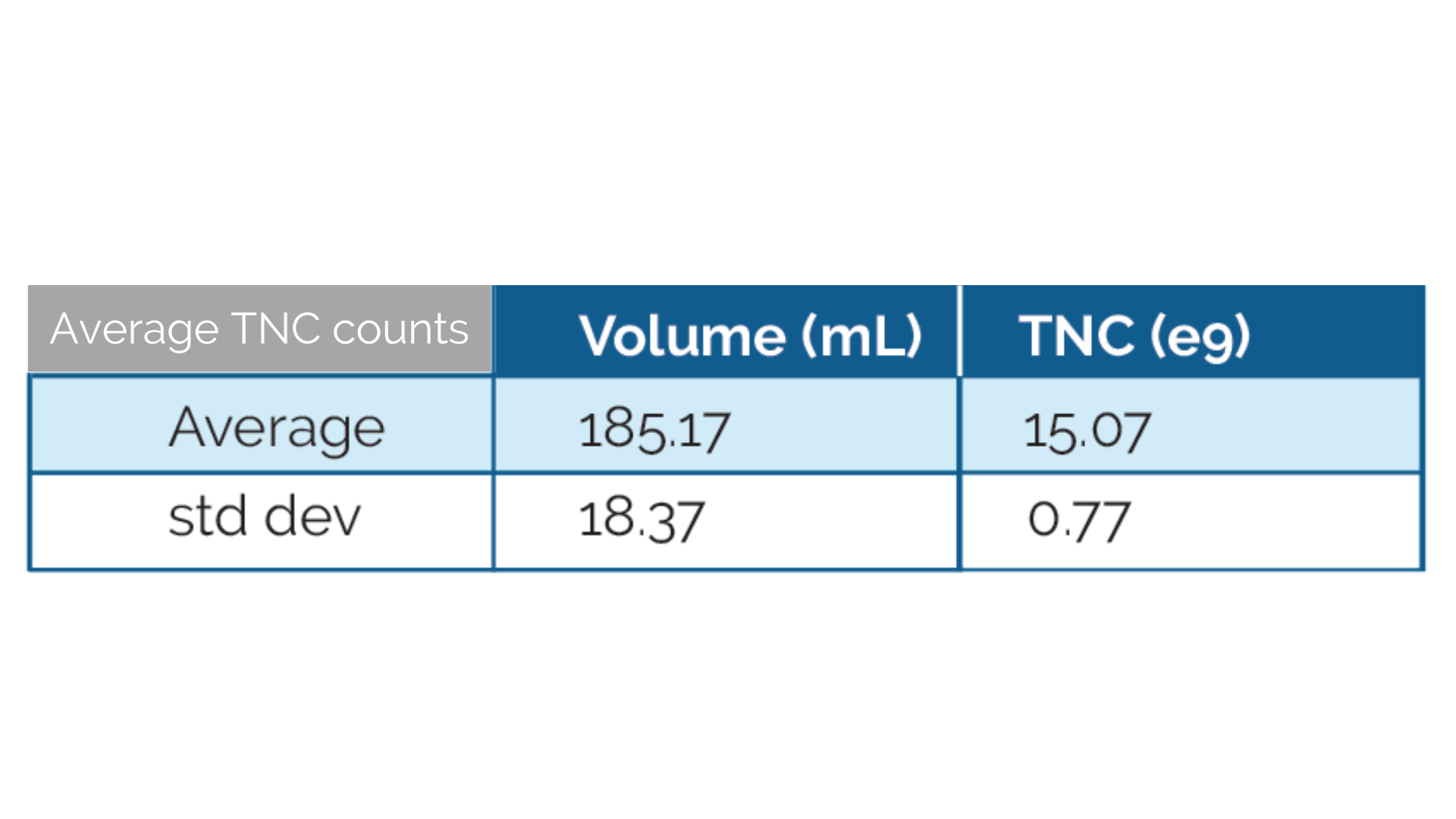
- Average 15 B TNC for full Leukopak
- Lower than 15% granulocytes
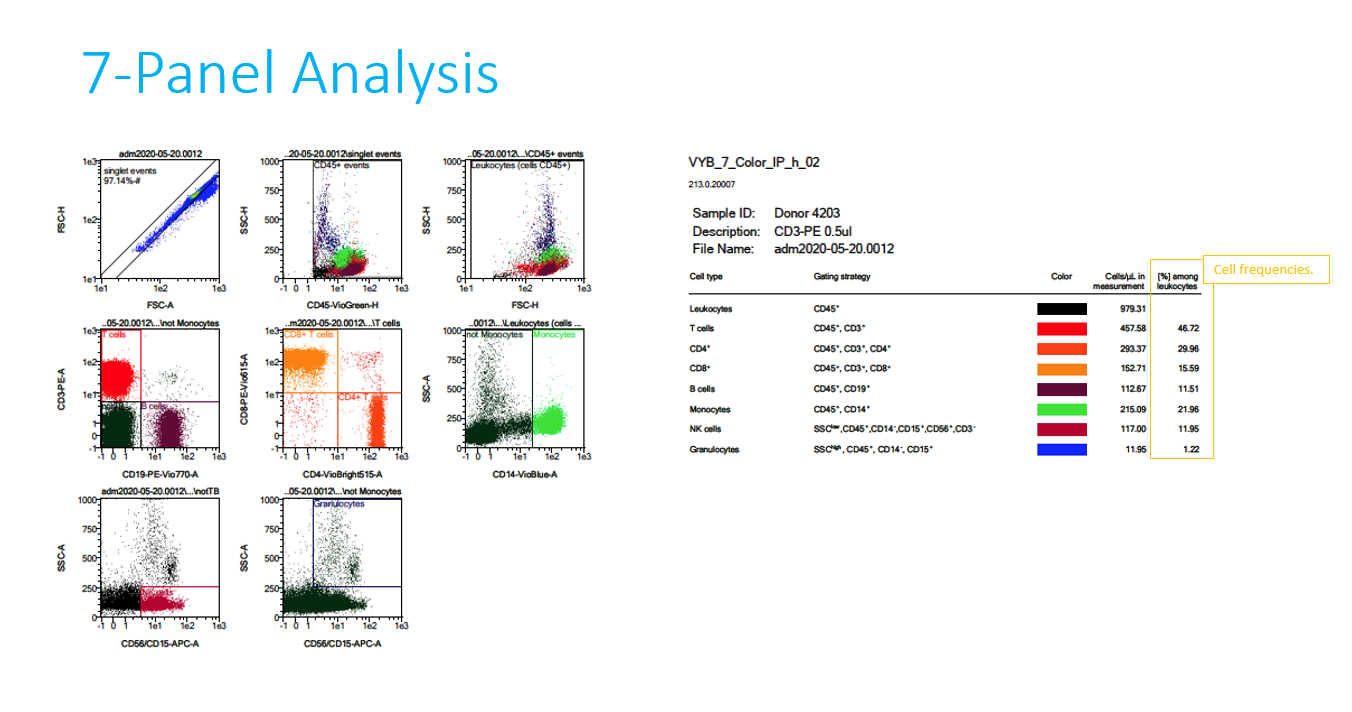
- 7 panel and CD34+ flow
- Recallable donors
- Bulk isolations available
- Up to 40ml of whole blood per Leukopak
- Same-Day Shipment from selected locations
- Special couriers for worry-free shipping
- Fresh and Cryopreserved Leukopaks available from the same collection
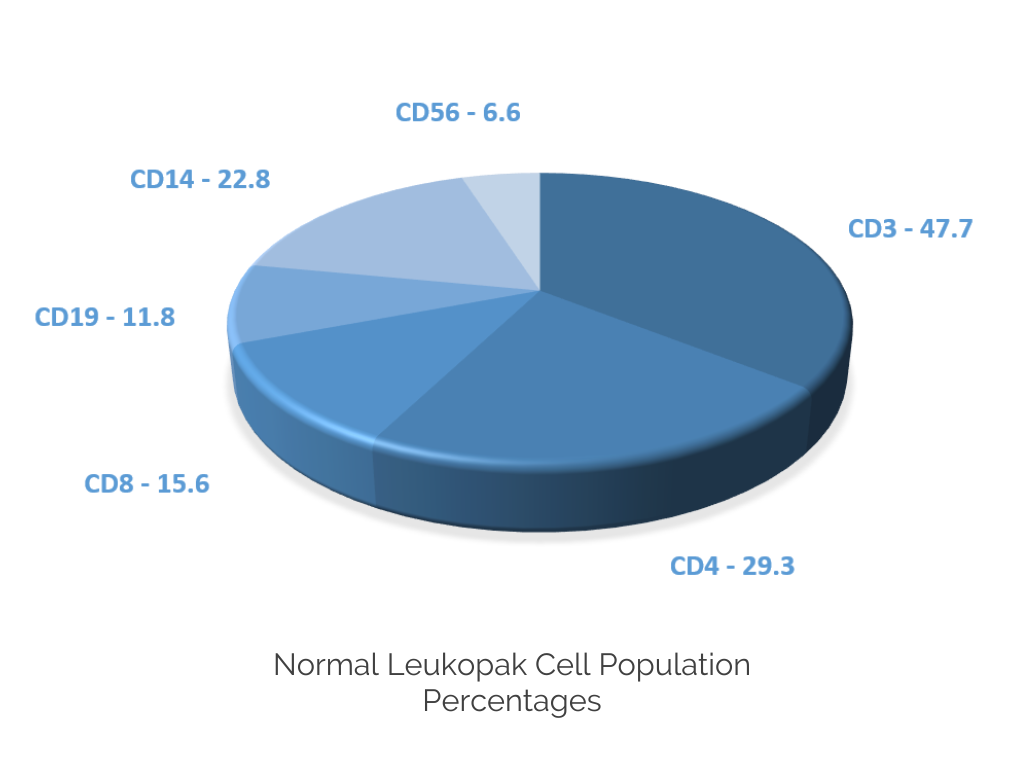
CGT Global Fresh Leukopaks have the following mean percentages of cell populations:
• T cells – 55%
• Monocytes – 27%
• B cells – 9%
• NK cells – 8%
• CD34+ stem cells – 0.1%
• Granulocytes – 2.4%
We do not guarantee the percentage of cell populations in a Fresh Leukopak, as they can vary from donor to donor.
Cells were obtained using Institutional Review Board (IRB) approved consent forms and protocols in CLIA Certified clinics registered by the FDA.
Custom Orders
We work closely with our customers to provide them with the product they need. For a custom order, please call and speak to one of our sales specialists at 530-303-3828.
Additional information
| Anticoagulant | |
|---|---|
| Format | |
| Grade | |
| Species | |
| Cell and Tissue Source | |
| Disease State | |
| Donor Attributes |
Product One-Sheet
Product Information Sheet
Certificate of Analysis
Material Safety Data Sheet
Publications
- Abelin et al. (2019) Defining HLA-II Ligand Processing and Binding Rules with Mass Spectrometry Enhances Cancer Epitope Prediction. Immunity 51(4): 766-779. Abstract