
From Product Development to Clinical and Commercial Production
The mission of CGT Global is to expedite the advancement of lifesaving therapies, from research and development to final drug approval and beyond. With 13 years’ experience as a leading provider of healthy donor blood products and primary cells, CGT Global supports the cell and gene therapy industry from research and pre-clinical through clinical trials and commercialization. As more cell and gene therapy candidates are moving through the drug development process, the need for clinical-ready partners to support these projects is critical to success. CGT Global provides products, services, and targeted donor recruitment from three privately owned locations nationwide and we are proud to offer our clients unparalleled flexibility, experience, partnership, cold chain logistics, and donor services to support your needs and improve patient outcomes around the globe.

Pre-Clinical (RUO) vs. Clinical (GMP) Products
Clients have different needs depending on their projects, and CGT Global consults with each client on an individual basis to determine compliance required based on the intended usage of our products. With more than a decade of experience as a leading provider of RUO and GMP healthy donor materials, CGT Global has unique expertise to provide customizable and flexible solutions to researchers and drug developers.
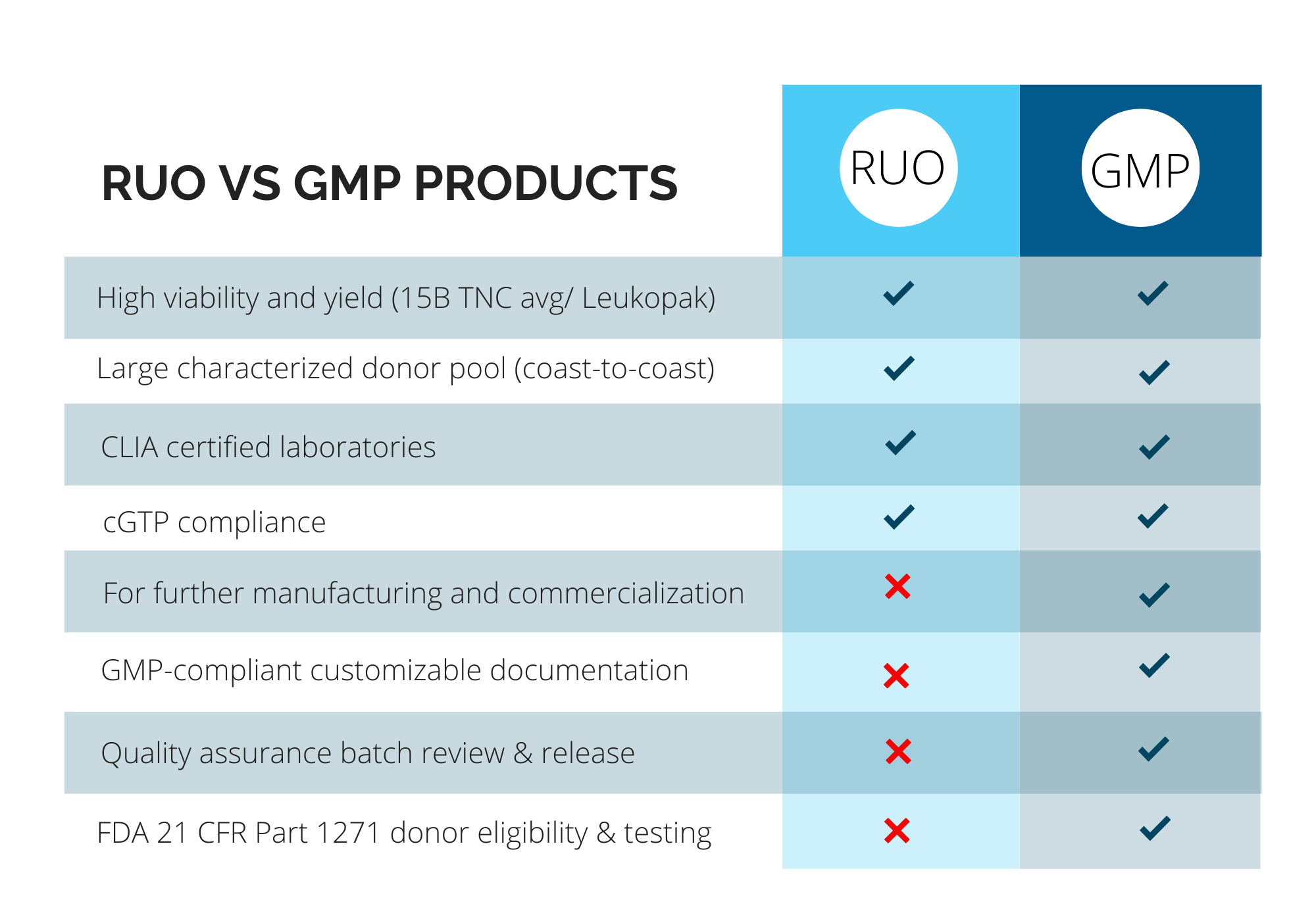
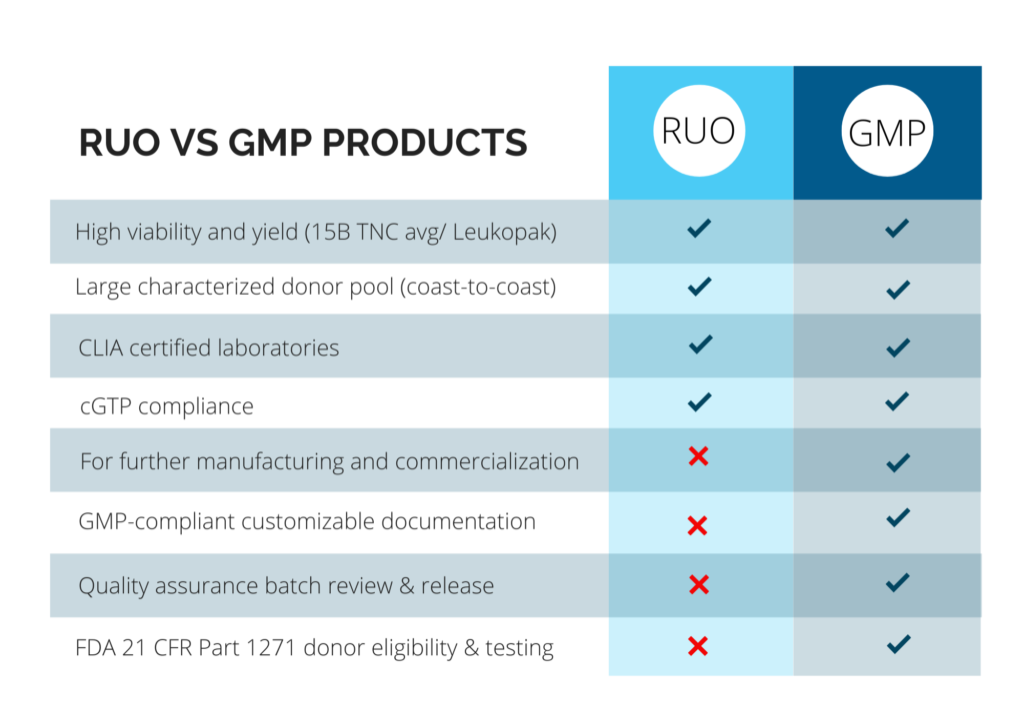
CGT Global adheres to local and federal regulations for clinical GMP-compliant products. We ensure donor and patient eligibility is determined according to FDA 21 CFR Part 1271 subpart C., and donor testing is performed within 7 days prior to each collection. We offer customized capabilities to meet additional or international regulatory requirements such as EMA regulations by adding on additional infectious disease testing at multiple time points, client-specific documentation, or other services to fit your project needs. GMP-compliant products must follow extensive quality assurance oversight and documentation in accordance with the FDA and other regulatory bodies. CGT Global employs a rigorous document control and training procedure under a compliant Quality Management System (QMS) and has written procedures in place for the release of product, process improvement and corrective and preventative actions.
Donor Recruitment Expertise and Services
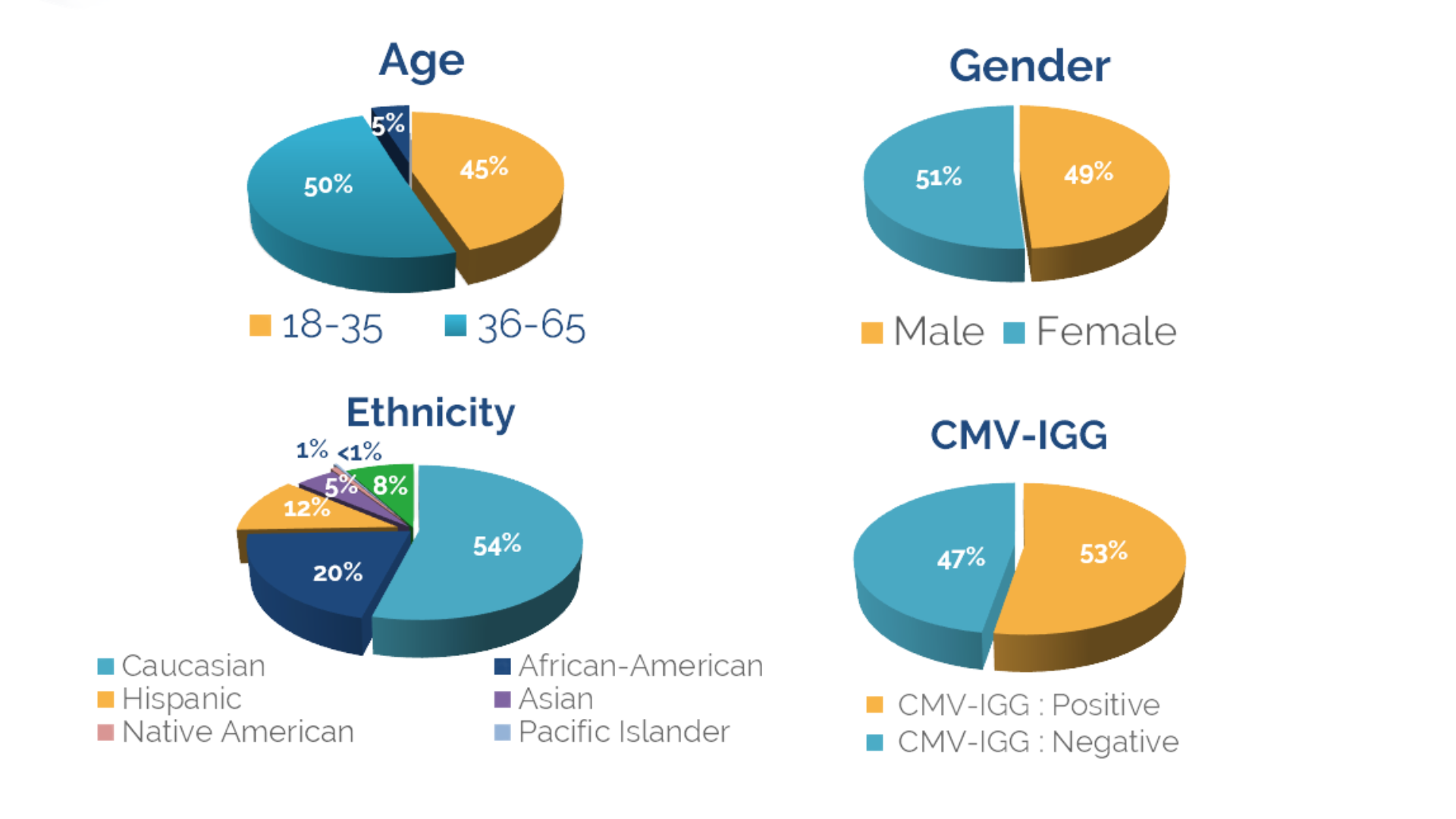
Healthy donors provide the source material for many advanced cell and gene therapies currently in development. Program success often depends on finding the right donor source material from a provider that can appropriately support the evolving clinical phase of a project. CGT Global offers unique services to identify donors based on data in our characterized donor pool. Additionally, CGT Global handles donor logistics ranging from screening for target genetic markers to customized IRB-approved consent forms or specific donor infectious disease testing needs.
Our recruitment team utilizes grassroots and community-based methods to engage with new donors, and actively seeks the best donors for allogeneic collections through a variety of donoration drives and local events. A dedicated recruiter and customer service representative on our team is available to manage client or program-specific donor pools.
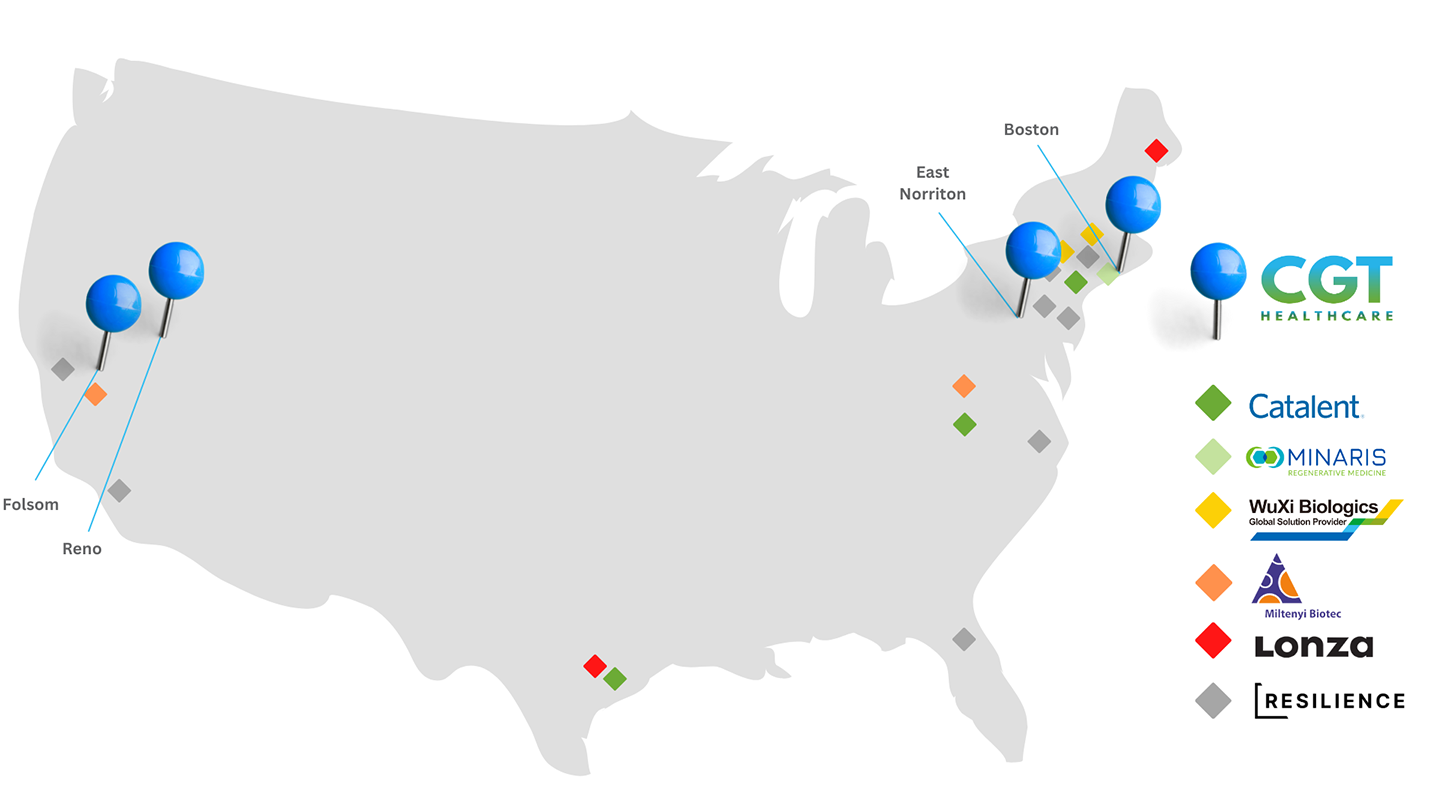
Map of CGT Global Locations and CDMOs
CGT Global has created a strategic network of cell collection centers with the cell and gene therapy industry in mind. Knowing that many therapeutic developers utilize third party manufacturing or CDMOs, we fulfill clinical collections intended for further manufacturing from all 6 collection facilities near major US-based CDMO partners. We can also work directly with your CDMO or manufacturing partner to simplify the fulfillment of our healthy donor products or patient collections. Utilizing our coast-to-coast footprint, CGT Global can mitigate supply risks caused by shipping delays, natural disasters, COVID closures, and other unforeseen circumstances.
High quality source material for the process and development phase of research
CGT Global offers a streamlined startup process and a flexible solution for GMP-compliant healthy donor starting material intended for further manufacturing. As you navigate through the startup and ordering process provided, CGT Global will collaborate with you directly to formulate a custom solution that will fit your specific needs all the way from donor recruitment to product fulfillment. Contact a representative to schedule an introductory or exploratory evaluation of your program.
Common process customizations:
- Location-specific donor collection in geographic proximity to your Manufacturing facility
- Complex donor screening and pre-qualification
- Specific donor recruitment or eligibility criteria
- Additional infectious disease testing (HHV6, 7, 8, Babesia, Toxoplasma, etc.)
- IRB-approved donor Informed Consent Forms (ICF)
- Supplemental Donor History Questionnaires (DHQ)
- Customized Certificate of Analysis (CoA)
- Analytical testing & other donor or collection characterization
- Shipping containers and carriers
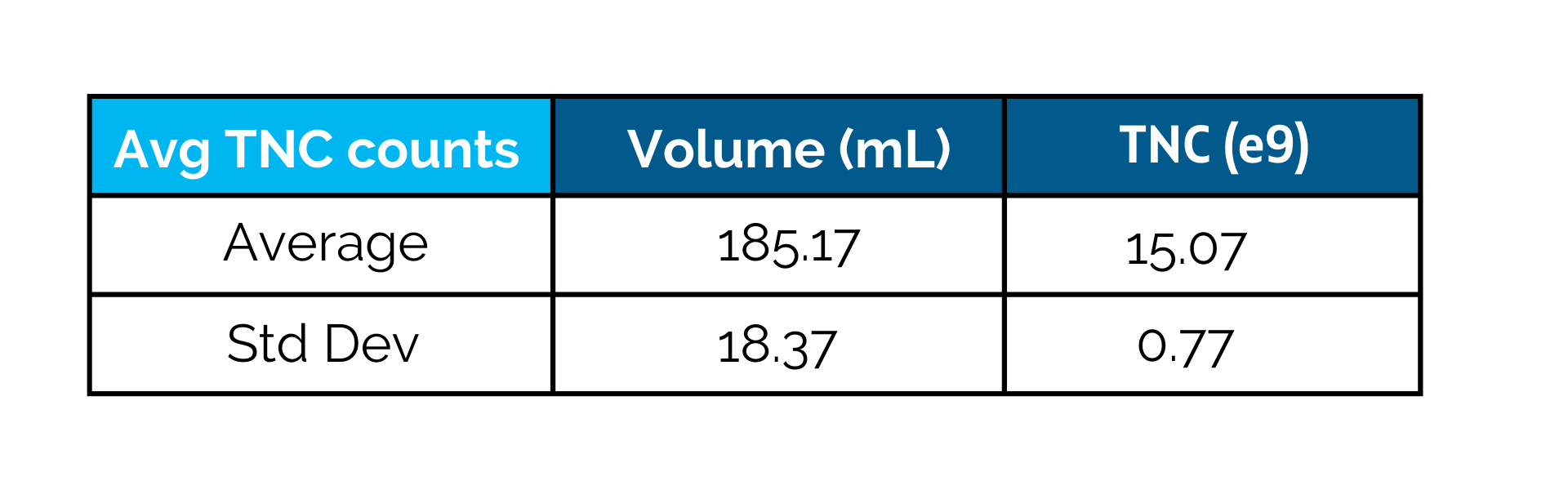
CGT Global Clinical Leukopaks are collected from IRB-approved consented donors or approved patients at 6 coast-to-coast FDA registered collection centers by qualified and trained staff using the Terumo Spectra Optia® cMNC collection protocol. Donor eligibility is determined following the AABB approved Donor History Questionnaire and in compliance with FDA 21 CFR subpart C infectious disease testing and medical history. CGT Global collection sites are FDA registered for HCT/P and our laboratories are CLIA registered. Standard infectious disease testing is performed by a qualified provider using FDA-licensed test methods. Collections are shipped fresh for same day, overnight or international delivery and documentation provided is customizable and compliant with GMP. CGT Global Quality Assurance reviews each batch for accuracy and release.
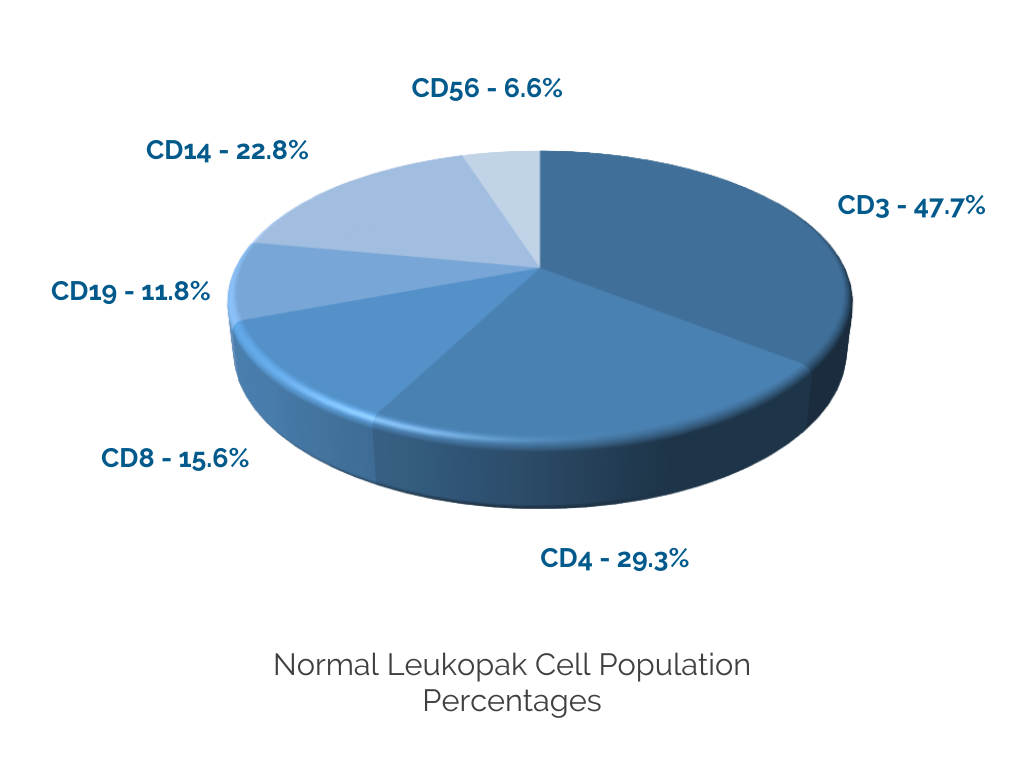
CGT Global collects Clinical Whole Blood at all collection sites by qualified and trained staff under an approved Batch Record using CPD anticoagulant bags. Donor eligibility is determined following the AABB approved Donor History Questionnaire and in compliance with FDA 21 CFR subpart C infectious disease testing and medical history. CGT Global collections are performed on-site at our CLIA registered and HCT/P FDA registered cell collection centers. A qualified provider performs standard infectious disease testing using FDA-licensed testing methods. Documentation can be customized to meet project needs and GMP compliance. CGT Global Quality Assurance reviews each batch for accuracy and release before product is shipped fresh for same day, overnight or international delivery.

Allogeneic cell and gene therapies such as CAR-T require the right donor and must often be selectively qualified and vetted prior to providing the raw material used in clinical manufacturing processes. This material is often subject to more extensive testing, and requires donors who are reliable and eligible for donation under 21 CFR 1271 subpart C if selected for clinical collection.
At CGT Global, we recognize this need in our industry and offer a unique product dedicated to finding the right donor on a program-specific basis and managed by our donor recruitment and collection staff accordingly.
.
This service includes: Leukapheresis, Whole Blood, Cryopreserved PBMCs
- FDA compliant Infectious Disease Testing panels
- Additional Infectious Disease Testing provided by approved vendors
- Donor characterization including HLA, KIR, genomic screening
- Location-based donor recruitment for supply chain optimization
- Dedicated donor management to your clinical program
- Further customization by evaluation


Clinical GMP Cleanroom Units
CGT Global proudly operates two BioSpherix Xvivo® X2 closed system ISO5 GMP units within its laboratory, fully outfitted to deliver clinical grade GMP compliant starting material for cell and gene therapy projects. The environment in the 2 suite BioSpherix Xvivo® X2 is calibrated to ensure optimal conditions for cells to thrive, including airflow, gas, and temperature regulation. This expertly designed compact cleanroom isolator allows laboratory technicians to operate freely within the laboratory, while never compromising samples within the unit through human error and contamination. The Xvivo® provides continuous audit tracking - monitoring particle count, environment, user logs, outages and errors - meaning full accountability at every single step.
The CGT Global mission is to help bring treatments and cures to patients at an accelerated pace. Utilizing this compact, efficient, highly effective technology, we can help clients scale therapies without the extreme overhead cost. CGT Global makes it possible for you to bring life-changing therapies to the end user, the patient, as a first line of defense against disease.

Closed System Cell Manipulation
CGT Global is proud to house the CliniMACS Prodigy® on-site. This device distills a full scale GMP laboratory into a single unit capable of performing cell isolations and manufacturing clinical grade products for pharmaceutical, therapeutic devices, allogeneic and autologous production. The closed system produces GMP grade cell isolations straight from apheresis without any exposure to external environmental contaminants, ensuring patient sample integrity is never compromised.
Through this groundbreaking device, CGT Global can partner with clients to run samples and create a cell therapy product, alleviating the need for hospitals to house and operate cumbersome laboratory equipment. This closed system processing is approved by the FDA for clinical use and Miltenyi offers multiple isolation kits, or custom technology can be uploaded onto the device as needed for specific projects under research partner’s or hospital’s regulatory compliance.


Your Complete Partner in Cell and Gene Therapy
"Our success is measured by the success of your projects - we help guide you every step of the way, so you can get your therapies to the patients who need them."
Cate Spears, Founder and CEO, CGT Global
Let's Take the Next Step
Download Our Clinical Capabilities Brochure
What You'll Learn About

LEARN MORE ABOUT CGT GLOBAL
We are the leading global biospecimen provider of human primary cells, stem cells, bone marrow, cord blood, peripheral blood, and disease-state products
Advancing Medical Breakthroughs

Advancing Medical Breakthroughs
Founded in 2010, we know how important biospecimens are to medical research and how frustrating it can be waiting for samples.
CGT Global is dedicated to accelerating research projects at life-changing speed to aid in the development of new treatments and even cures for patients worldwide.

