CGT Global Products
GMP Products
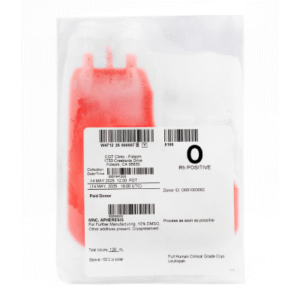
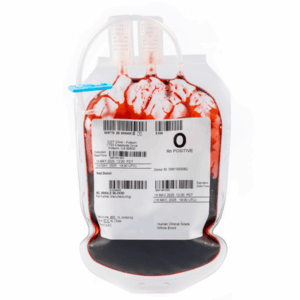
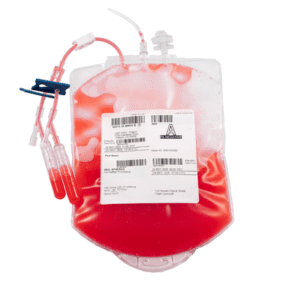
CGT Global GMP Products are GMP-compliant and intended for use in Cell Therapy manufacturing processes.
GMP Leukopaks are and collected from IRB-approved consented healthy donors at FDA registered clinics by qualified and trained staff using the Terumo Spectra Optia cMNC collection protocol.
We determine donor eligibility following the AABB approved Donor History Questionnaire for Hematopoietic Progenitor Cells (HPC-DHQ). Donor eligibility is in compliance with FDA 21 CFR Part 1271 subpart C for infectious disease testing and physical examination.
Showing all 3 resultsSorted by popularity