Fresh Leukopaks
Since our founding in 2010, CGT Global has pursued our mission to transform healthcare as we accelerate cell and gene therapy research and clinical trials, streamline the commercialization of new treatments, and map the last mile to patient access to these life-changing remedies. By innovating each stage in the cycle; development, commercialization, and delivery, we reduce the overall cost of the care and multiply access points so that millions can receive cutting edge, life-saving gene and cell therapies.

Description
CGT Global Fresh Leukopaks are collected from healthy IRB consented donors, that have been tested negative for HBV, HCV, and HIV. Leukopaks are collected using the Spectra Optia® Apheresis System, where cell-rich mononuclear cells are extracted using a continuous flow system directly into a sterile collection bag containing the anticoagulant ACD-A. CGT Clinics are CLIA Certified and FDA Registered. Up to 12 Fresh Leukopak collections can be performed on the same donor in a given year, providing an ample amount of PBMCs for large-scale experiments and research studies. Additionally, 200-300mL of plasma can be collected at the time of the donor draw.
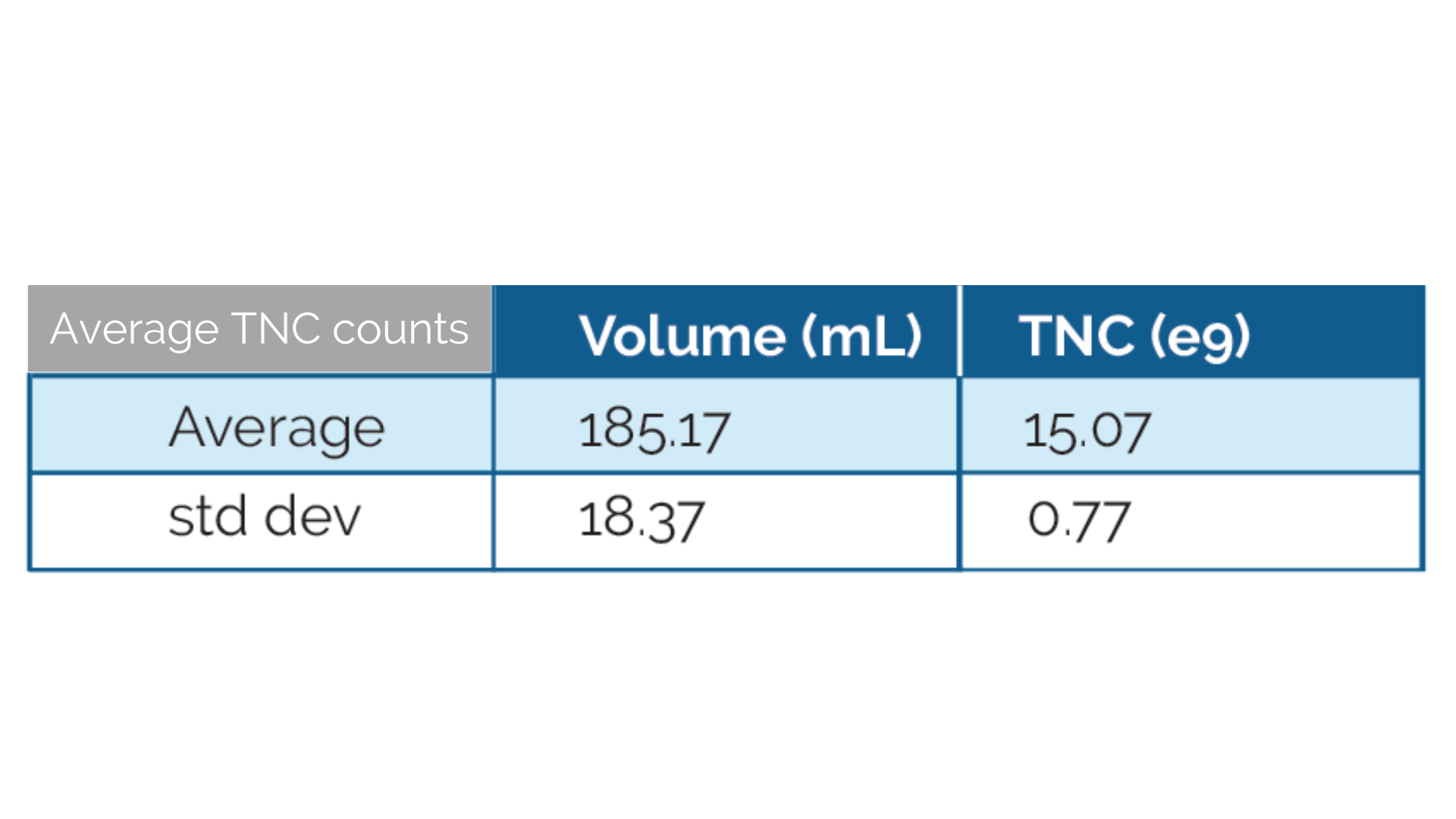
- Average 15 B TNC for full Leukopak
- Lower than 15% granulocytes
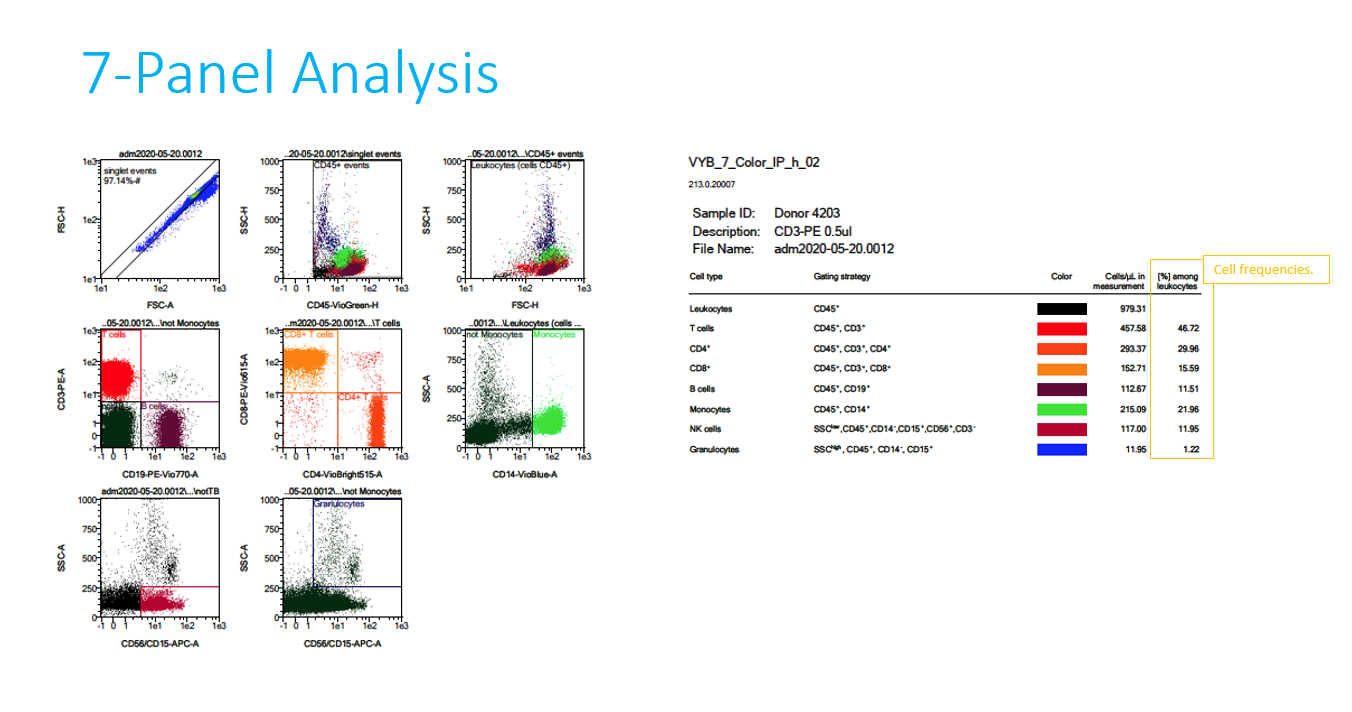
- 7 panel and CD34+ flow
- Recallable donors
- Bulk isolations available
- Up to 40ml of whole blood per CGT Global Leukopak
- Same-Day Shipment from selected locations
- Special couriers for worry-free shipping
- Fresh and Cryopreserved Leukopaks available from the same collection
- Disease State Leukopaks available
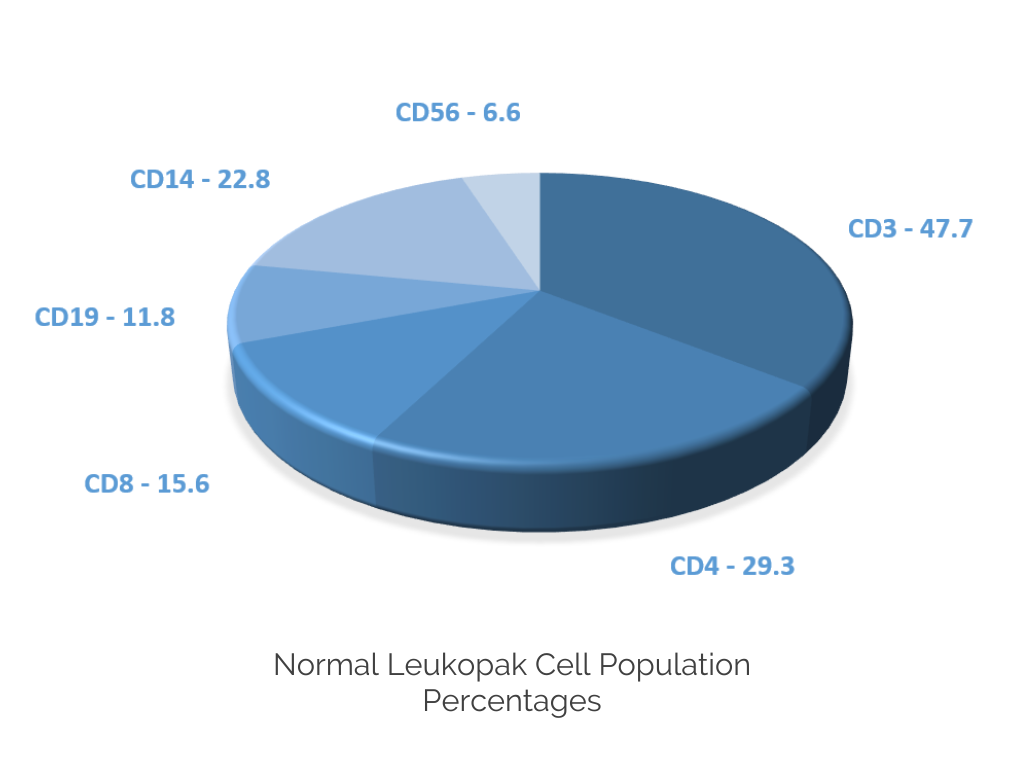
CGT Global Fresh Leukopaks have the following mean percentages of cell populations:
• T cells – 55%
• Monocytes – 27%
• B cells – 9%
• NK cells – 8%
• CD34+ stem cells – 0.1%
• Granulocytes – 2.4%
We do not guarantee the percentage of cell populations in a Fresh Leukopak, as they can vary from donor to donor.
Cells were obtained using Institutional Review Board (IRB) approved consent forms and protocols in CLIA Certified clinics registered by the FDA.
Custom Orders
We work closely with our customers to provide them with the product they need. For a custom order, please call and speak to one of our sales specialists at 530-303-3828.
Additional information
| Anticoagulant | |
|---|---|
| Format | |
| Grade | |
| Species | |
| Cell and Tissue Source | |
| Disease State | |
| Donor Attributes |
Product Information Sheet
Material Safety Data Sheet
Publications
- Abelin et al. (2019) Defining HLA-II Ligand Processing and Binding Rules with Mass Spectrometry Enhances Cancer Epitope Prediction. Immunity 51(4): 766-779. Abstract