By: Joanna Wirkus
Estimated reading time: 8 minutes
Table of contents
The Rise of Cord Blood CD34+ Cells in Humanized Mouse Models and Cell Therapy Innovation
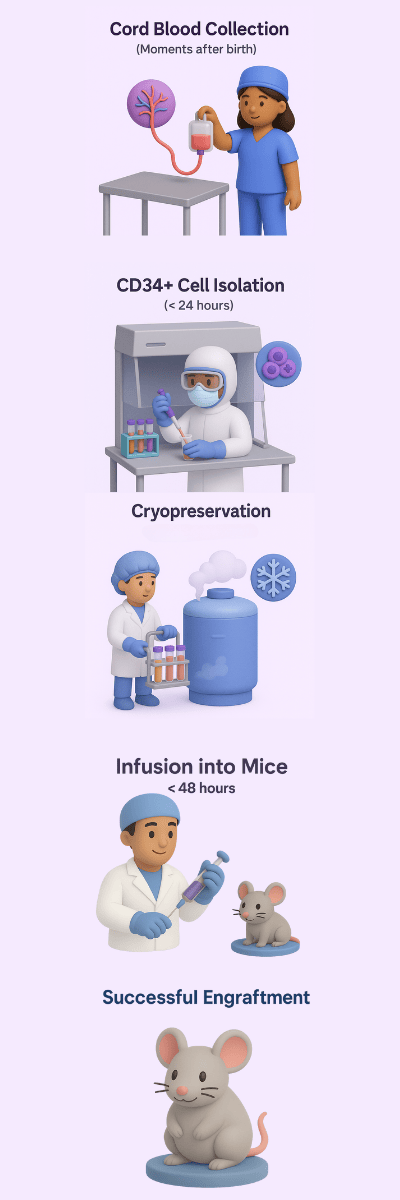
Scientists in the late 1980’s discovered that umbilical cord blood derived stem cells had high proliferative potential and tolerated cryopreservation and thawing (Broxmeyer, 1989). Building on this research, the first report of cord blood derived CD34+ human stem cells successfully engrafting and restarting hematopoiesis in immunodeficient mice was published in 1997 (Hogan, 1997). Placenta and umbilical cords were previously discarded as medical waste. Today, they are a noninvasive alternative to bone marrow and have proven to be a rich source of primary cells for cell and gene therapy research. Cord blood derived naive stem cells carry a lower risk of graft-vs-host disease which supports the development of high numbers of consistent humanized mice for research studies. Mice with a human immune system engrafted by cord blood derived CD34+ cells are an important advancement in in-vivo research in oncology, immunology, and endocrinology.
For more on humanized mice models, see our blog “Overcoming Challenges with Animal Models in Research” on this topic.
At CGT Global, we have years of experience collecting cord blood and isolating primary cord blood derived cells to accelerate cell and gene therapy research. With this expertise, we are proud to offer high quality and customizable whole and isolated cord blood products to our research community.
Cell viability is directly linked to graft potency. To provide scientists with high viability cells we optimize temperature, blood volume, and time during the workflow from blood collection to cell isolation. Our standard operating procedures are rooted in decades of research on optimizing cell collection and processing methods. Numerous research studies have demonstrated that time from collection to processing is the top factor influencing cell viability in donated cord blood (Dulgiac, 2014; Fry, 2012; Bouchony, 1993). Some researchers have also established that total volume of blood collected can influence cell viability, although time to processing has proven to be the number one optimizable factor for attaining the highest possible cell viability (Pope, 2012).
Why choose CGT Global?
Achieving high engraftment rates in humanized mouse models is dependent on high viability CD34+ cells and high and accurate cell counts. Scientists trust us to provide high quality, reliable starting materials with our optimized protocols from collection to delivery. We maintain strict compliance to a wide array of regulatory requirements including IRB, FDA, and CLIA, etc. Quality assurance measures are implemented from start to finish so our customers’ satisfaction is 100% guaranteed.
How do we do it? We provide consistent, high-quality cells thanks to our:
Highly skilled staff
- CGT Global’s cord blood collection technicians have specialized medical and phlebotomy training and years of experience with collecting cord blood immediately after birth. They are skilled at collecting the maximal amount of blood from the cord and placenta using evidence-based and standardized protocols to provide the highest cell viability. Our team works directly in the hospitals, 24 hours a day, to ensure the cord blood is collected as soon as possible.
- In our laboratory, our cell scientists follow tested and standardized protocols for optimizing cell processing for consistent results. We include infectious disease testing for all our products and can provide additional information such as donor demographics or blood type and typing upon request. Isolated cells can be from a single source donor or from pooled donors.
Streamlined logistics cord blood collection and processing
- Dedicated technicians are on-staff at hospitals ready to consent donors and collect cord blood 24 hours a day, 7 days a week, 365 days a year. Our time-tested biospecimen collection and transportation logistics allow us to process cord blood within hours of delivery. With highly available staff and a short distance from the hospitals to our laboratory, we have minimized blood collection to cell processing time to optimize cell viability.
- Other stem cell sources may rely on left over blood products that have been stored for a prolonged amount of time and thus have lower quality cells or don’t meet the minimum qualifications for cord blood banking so they are a low quality starting material.
Strategic infrastructure for shipping finished cell products
- Successfully distributing cells to our worldwide network is our area of expertise. We reliably deliver cell products locally and around the globe.
- Depending on the location, some orders can be delivered on the same day.
- Our cell processing labs are strategically located in Folsom, CA, and Cambridge, MA, and East Norriton, PA near major airports for smooth shipments to international and domestic laboratoriess.
- Shipment options are customizable for your next experiment, whether you need your starting materials to arrive fresh or frozen.
- Temperature and location are tracked throughout the shipment with updates provided to the sender and receiver.
Customizable – Your cells, your way!
- We offer whole cord blood, cord blood plasma, isolated primary cells, and numerous customizable cell isolations including multiple cell types from a single donor
- We can provide fresh or frozen whole or isolated biospecimens
- Cells are 100% guaranteed to have high viability, high purity, and accurate cell count
- We offer large and customizable lot sizes from single or pooled donors
- Freezing media can be standard or custom
One of our most popular isolated cord blood cell types are CD34+ stem/progenitor cells
- CD34+ Stem/Progenitor cells isolated from Cord Blood
- These cells are popular for humanized mouse models and our quality cells yield the highest engraftment rates in the industry. They have been used by scientists to better model human immune responses (Halkais, 2015), study therapeutic approaches to address food allergies (Ito, 2021), and to develop gene editing interventions for congenital megakaryocytic thrombocytopenia (Cleyrat, 2017).
CGT Global’s expert staff have processed over 9,000 units of cord blood since 2021. As the only cell supplier in the industry with the capacity to perform end-to-end cord blood collection, CGT Global’s staff is committed to providing the best possible cell products to accelerate medical discoveries. We combine multidisciplinary skillsets to deliver high quality starting materials for scientists with rigorous quality assurance throughout the entire process. For example, our talented lab technician recently isolated 20 million CD34+ from a single cord blood unit! Having a consistent source of stem cells for creating humanized mice eliminates donor to donor variation and facilitates reproducible follow up studies.
“Our talented lab technician recently isolated 20 million CD34+ from a single cord blood unit!”
CGT Global lab record
Let us take care of the logistics of collecting and curating cord blood cells so you can focus on the science that can change lives. By leveraging our strengths together, we can bring better science forward and deliver hope to patients by turning cells into cures.
Contact us today to learn how CGT Global can elevate your research on human cells with our world-class cord blood biomaterials. Call us at 530-303-3828 or contact us online!
Cord Blood FAQ’s
CD34+ stem cells are hematopoietic progenitor cells found in cord blood, bone marrow, and peripheral blood. They play a critical role in blood and immune system development. In research, they’re used for studying hematopoiesis, gene editing, and creating humanized mouse models for immunotherapy and infectious disease studies.
Cord blood offers a rich, naïve source of CD34+ cells with lower immunogenicity compared to adult sources. This makes them ideal for humanized mice, where reduced graft-versus-host disease (GVHD) risk and high engraftment rates are essential.
CGT Global uses advanced positive or negative immunomagnetic isolation methods. Our isolations are highly customizable and follow rigorous quality control protocols to ensure high purity and viability.
Our CD34+ isolations typically exceed 95% purity and 92% viability, making them ideal for demanding applications like CRISPR/Cas9 editing, xenotransplantation, and CAR-T preclinical validation.
Absolutely. CGT Global offers extensive donor characterization, including HLA-A typing, CMV status, age, ethnicity, and more. Custom recruitment is available to match your specific study needs.
We offer both fresh and cryopreserved CD34+ cells. Same-day processing and shipping are available from our coast-to-coast U.S. facilities, with coordinated logistics for global delivery. Most custom orders ship within 3–7 business days of placing the order and receive your order 48 hours from collection.
References
- Broxmeyer, H.E., Douglas, G.W., Hangoc, G., Cooper, S., Bard, J., English, D., Arny, M., Thomas, L., and Boyse, E.A. (1989). Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci USA 86, 3828–3832. 10.1073/pnas.86.10.3828.
- Hogan, C.J., Shpall, E.J., McNulty, O., McNiece, I., Dick, J.E., Shultz, L.D., and Keller, G. (1997). Engraftment and development of human CD34(+)-enriched cells from umbilical cord blood in NOD/LtSz-scid/scid mice. Blood 90, 85–96. 10.1182/blood.V90.1.85.
- Dulugiac, M., Horeanga, I., Torcatoru, A., Bardas, A., Matei, G., and Zarnescu, O. (2014). Factors which can influence the quality related to cell viability of the umbilical cord blood units. Transfus. Apher. Sci. 51, 90–98. 10.1016/j.transci.2014.08.019.
- Fry, L.J., Giner, S.Q., Gomez, S.G., Green, M., Anderson, S., Horder, J., McArdle, S., Rees, R., and Madrigal, J.A. (2013). Avoiding room temperature storage and delayed cryopreservation provide better postthaw potency in hematopoietic progenitor cell grafts. Transfusion 53, 1834–1842. 10.1111/trf.12006.
- Tron de Bouchony, E., Pelletier, D., Alcalay, D., Bruneau, J., Predeau, M., Brizard, A., and Magnin, M. (1993). Hematopoietic progenitor content of fetal cord blood collected using citrate-phosphate-dextrose: influence of holding temperature and delays. J. Hematother. 2, 271–273. 10.1089/scd.1.1993.2.271.
- Pope, B., Mitsakos, K., Bilgin, A., Hokin, B., and Grant, R. (2012). Predicting overall viability of cord blood harvests. Transfusion 52, 1079–1085. 10.1111/j.1537-2995.2011.03386.x.
- Halkias, J., Yen, B., Taylor, K.T., Reinhartz, O., Winoto, A., Robey, E.A., and Melichar, H.J. (2015). Conserved and divergent aspects of human T-cell development and migration in humanized mice. Immunol. Cell Biol. 93, 716–726. 10.1038/icb.2015.38.
- Ito, R., Katano, I., Otsuka, I., Takahashi, T., Suemizu, H., Ito, M., and Simons, P.J. (2021). Bovine β-lactoglobulin-induced passive systemic anaphylaxis model using humanized NOG hIL-3/hGM-CSF transgenic mice. Int. Immunol. 33, 183–189. 10.1093/intimm/dxaa067.
- Cleyrat, C., Girard, R., Choi, E.H., Jeziorski, É., Lavabre-Bertrand, T., Hermouet, S., Carillo, S., and Wilson, B.S. (2017). Gene editing rescue of a novel MPL mutant associated with congenital amegakaryocytic thrombocytopenia. Blood Adv. 1, 1815–1826. 10.1182/bloodadvances.2016002915.