Since our founding in 2010, CGT Global has pursued our mission to transform healthcare as we accelerate cell and gene therapy research and clinical trials, streamline the commercialization of new treatments, and map the last mile to patient access to these life-changing remedies. By innovating each stage in the cycle; development, commercialization, and delivery, we reduce the overall cost of the care and multiply access points so that millions can receive cutting edge, life-saving gene and cell therapies.

Description
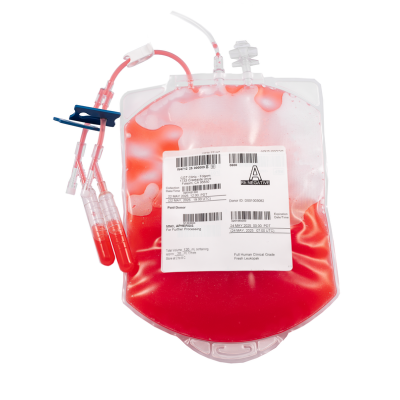
Fresh GMP Leukopaks are collected to target a minimum 10 Billion TNC yield and ≥ 90% viability and are shipped fresh. Overnight or international delivery and documentation provided is customizable and compliant with GMP. CGT Global Quality reviews each batch for accuracy and release.
GMP Leukopaks are and collected from IRB-approved consented healthy donors at 6 coast-to-coast FDA registered collection centers by qualified and trained staff using the Terumo Spectra Optia cMNC collection protocol. We determine donor eligibility following the AABB approved Donor History Questionnaire for Hematopoietic Progenitor Cells (HPC-DHQ). Donor eligibility is in compliance with FDA 21 CFR Part 1271 subpart C for infectious disease testing and physical examination.
CGT Global laboratories are FDA and CLIA registered. Our standard infectious disease testing are performed by a qualified provider using FDA-licensed test methods.
For more information or a custom project evaluation, request more information.
Additional information
| Anticoagulant | |
|---|---|
| Format | |
| Grade | |
| Species | |
| Cell and Tissue Source | |
| Disease State | |
| Donor Attributes | T. Cruzi (Chagas) Ab, CMV Total, CMV Total with Reflex to IgM (Only performed if CMV total test result is positive), HIV I & II Ab + Reflex, CMV Total with Reflex to IgM and IgG/IgM (Only performed if CMV total test result is positive), HIV/HBV/HCV NAT, Zika NAT* inquire, HCV Ab, Additional Testing* inquire, HBV Ab Core + Reflex, HBV Ab Surface Ag + Reflex, HTLV I & II Ab + Reflex, Syphilis Ab, West Nile Virus NAT |