CGT Global Products
Leukopaks
CGT Global Leukopaks are collected at 3 FDA registered CGT Clinics, providing ample peripheral blood mononuclear cells (PBMCs) for large scale experiments. Request a quote for fresh or frozen leukopaks to support your project needs.
Leukopaks: Fresh | Frozen | Mobilized
CGT Globals' Peripheral Blood Leukopaks provide enriched peripheral blood mononuclear cells (PBMCs) with low granulocyte and red blood cells. This highly enriched PBMC fraction requires minimal downstream processing (i.e. Ficoll) and can be used directly for isolating high yields of immune cells, such as CD4+ T cells, CD8+ T cells, CD19+ B cells, monocytes, and other immune cells. High cell yields allow researchers greater reproducibility of experiments from a single donor (minimizing donor-to-donor variation), a decrease in same cell variability, and scalability of experiments. In addition, up to 12 Leukopaks can be collected from the same donor in a given year, providing an ample amount of PBMCs for large-scale experiments and research studies. CGT Global also offers Disease State Leukopaks for research.
Leukopaks are sent out on the same day or frozen immediately to ensure a highly viable product.
- 1x1010TNCs/full bag
- Highly-enriched PBMCs
- ≥95% viability (fresh)
- HBV, HCV, HIV negative
- IRB approved
What are Leukopaks?
A leukopak, short for leukapheresis pack, is a concentrated amount of white blood cells (leukocytes) collected from a donor to be used in research or even medical procedures. Leukopaks are typically obtained through leukapheresis, a process where blood is drawn from the donor, passed through an apheresis machine that separates out the white blood cells, and then returns the remaining blood components to the donor's circulation. Leukopaks are commonly used in medical research, particularly in studies involving immunology, cell therapy, and vaccine development, where a high concentration of white blood cells is required for experimentation or treatment.
Benefits of Leukopaks
- Higher WBC concentration than whole blood
- Superior purity
- Source of specific cell types

CGT Global Donors
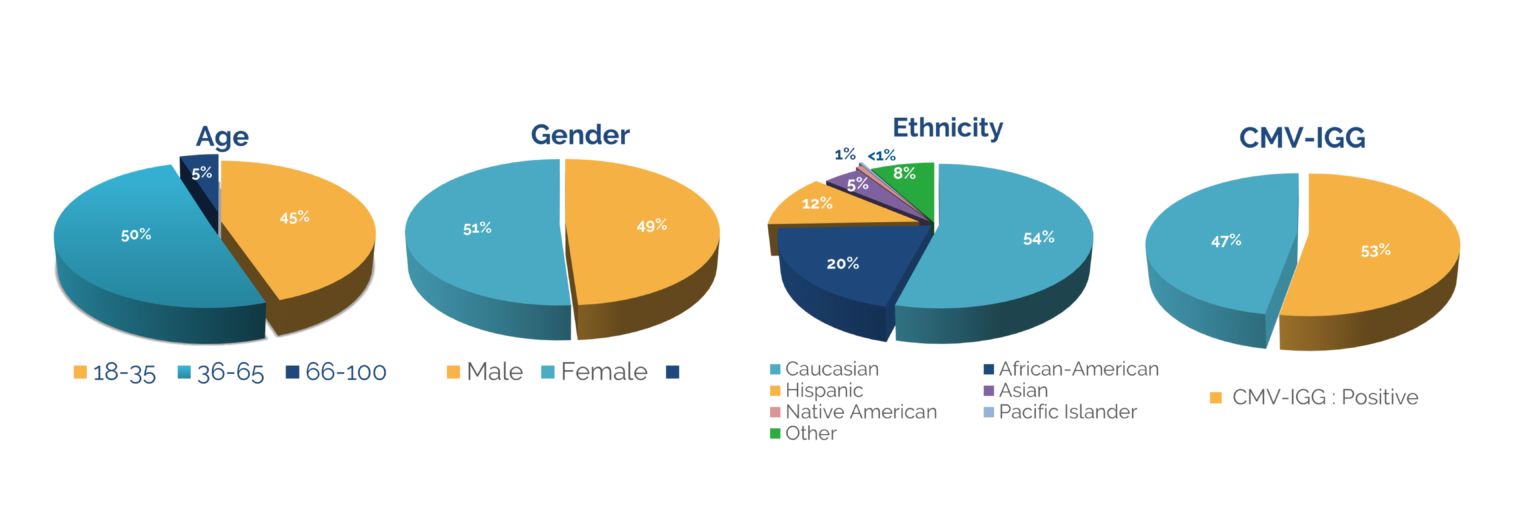
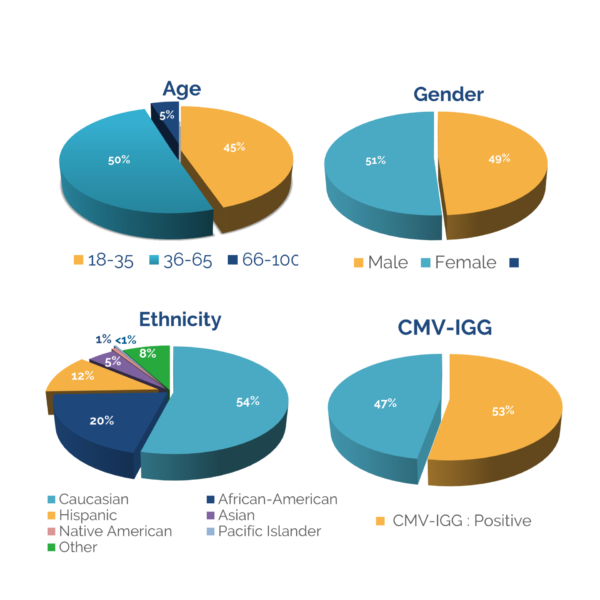
Access to a diverse donor pool is key to the successful development of new therapeutics. With 3 locations nationwide, the CGT Global donor pool is one of the largest HLA-typed and most characterized in the industry. All collection centers are FDA registered, and boast on-site state-of-the-art laboratories, providing an end-to-end service whereby cells are processed, isolated, and cryopreserved or shipped out fresh immediately after collection from a donor.
Choosing a complete partner in research starts with a reliable donor base - from demographic diversity and targeted recruitment to recallability and retention. CGT Global focuses on finding the right donors for a project, with customizable IRB consents and downstream protocols to fit the client's needs. Once a donor is fit to a project, CGT Global works diligently to ensure recallability, with expert donor retention strategies in place.
Donors are an integral part of clinical and preclinical project planning. Knowing how accessible your target donors are, and your ability to reliably recall them can indicate future success and scalability of a project. CGT Global strategically maps out each step in the donor/research process, handling logistics ranging from screening for target genetics, to customizing IRB consents.
In addition to managing donor specifications, CGT Global provides access to a coast-to-coast donor network, with a wide, demographically diverse footprint. Given our far-ranging national reach, CGT Global is able to mitigate supply risks caused by natural disasters, COVID closures, and unforeseen shipping delays.
Our recruitment team seeks out the best donors by engaging with our communities through local businesses, fairs, restaurants, colleges, health and wellness expos, gyms, and donor drives.
- Broad and/or targeted donor recruitment efforts across 5 states
- Collections take place in safe, clean, private collection rooms
- Highly effective donor retention protocols
- Strict guidelines and quality standards - ensuring consistent, compliant, viable, and pure cells with guaranteed counts
- Local same-day delivery within 150 miles
- Recallable and repeat donors
- Customizable donor/patient questionnaire
- Specific donor demographics (age, weight, ethnicity, etc.)
- Donor lifestyle characteristics (BMI, smoker/non-smoker, etc.)
- Medical history (allergies, medications, etc.)
- HLA, RhD, and ABO typing available
- Additional infectious disease screening (CMV, etc.)
- Global reach & delivery
- Good Tissue Practices (GTP)
- All sites FDA Registered

Apheresis
Apheresis is a critical starting point for all research projects, setting the standard for downstream project success through high-quality starting material. CGT Global is a leading provider of apheresis products, with an expansive footprint through our coast-to-coast CGT clinics and unprecedented access to demographically diverse donors. Our expertly trained technicians ensure every draw is GMP compliant, and subsequently shipped out fresh or cryopreserved on-site.
CGT Global collections take place in clean, private, individual collection rooms ensuring a controlled and comfortable experience for donors. Leukopaks are collected in a highly regulated FDA-approved closed system Spectra Optia® Apheresis machine using continuous auditing.
Apheresis collections can be sent to the researcher as a fresh product or sent to the CGT Global lab to be further isolated, or manufactured through custom protocols under the research partner’s or hospital’s regulatory compliance. CGT Global also offers bulk cell isolations from Leukopaks.
Leukapheresis Procedure
Leukopaks are typically collected through a process called leukapheresis that includes the following steps:
- Donor Screening: Potential donors are screened for eligibility based on health criteria to ensure they are suitable for donation. This involves assessing their medical history, current health status, and specific laboratory tests.
- Preparation: Before the leukapheresis procedure, donors receive instructions on fasting or hydration to prepare for the donation.
- Access: The donor is connected to an apheresis machine via one or more intravenous lines. The machine is equipped with sterile tubing and specialized filters.
- Blood Collection: Blood is drawn from the donor and circulated through the apheresis machine. Inside the machine, the blood is separated into its components using centrifugation or filtration techniques in the setting of a leukopak donor center.
- White Blood Cell Collection: The apheresis machine selectively removes the white blood cells from the donor's blood. This process involves separating white blood cells from other blood components based on their physical properties, such as size or density.
- Return of Remaining Blood Components: After the white blood cells have been collected, the rest of the blood, including red blood cells, platelets, and plasma, is returned to the donor's circulation. This helps minimize the impact of the donation on the donor's overall blood volume.
- Collection and Storage: The collected leukopak, containing a concentrated amount of white blood cells, is processed further as needed and then stored under controlled conditions until it is ready for use in research or medical applications.
Overall, leukapheresis allows for the selective collection of white blood cells from a donor while returning the other blood components to the donor's circulation, making it a valuable tool in medical research and therapies requiring a high concentration of white blood cells.

A fresh leukopak refers to a leukopak that has been collected and processed without undergoing significant storage or preservation methods. Essentially, it's a leukopak that is used soon after collection, typically within a short period of time.
Fresh leukopaks are often preferred in research and medical applications where the viability and functionality of white blood cells are critical. This is because white blood cells can be sensitive to storage conditions and may experience changes in activity or function over time.
Researchers and clinicians may opt for fresh leukopaks when they require high-quality, active white blood cells for experiments, treatments, or therapies. For example, in cellular immunotherapy, fresh leukopaks containing active immune cells are often used for the isolation, expansion, and manipulation of specific cell populations for therapeutic purposes.
Fresh leukopaks are collected through leukapheresis procedures, and after collection, they may undergo minimal processing steps before being utilized in research or medical applications. This ensures that the white blood cells remain in a highly functional state and suitable for their intended purpose.
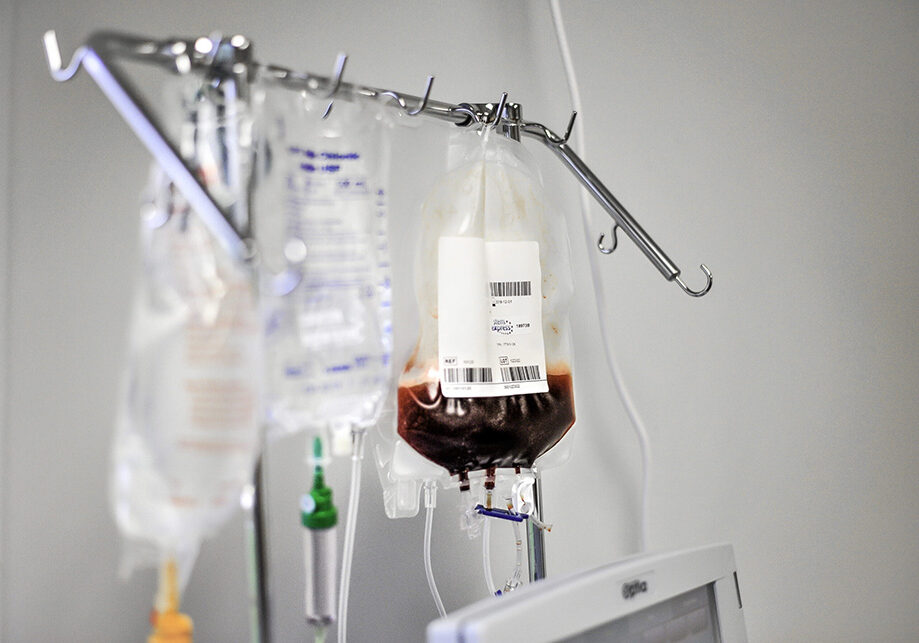
Leukopaks are collected from healthy IRB consented donors, tested negative for HBV, HCV, and HIV, using the Spectra Optia® Apheresis System, where cell-rich mononuclear cells are extracted using a continuous flow system directly into a sterile collection bag containing anticoagulant. Up to 12 Leukopak collections can be performed on the same donor in a given year, providing an ample amount of PBMCs for large-scale experiments and research studies. Additionally, 200-300mL of plasma can be collected at the time of the donor draw.
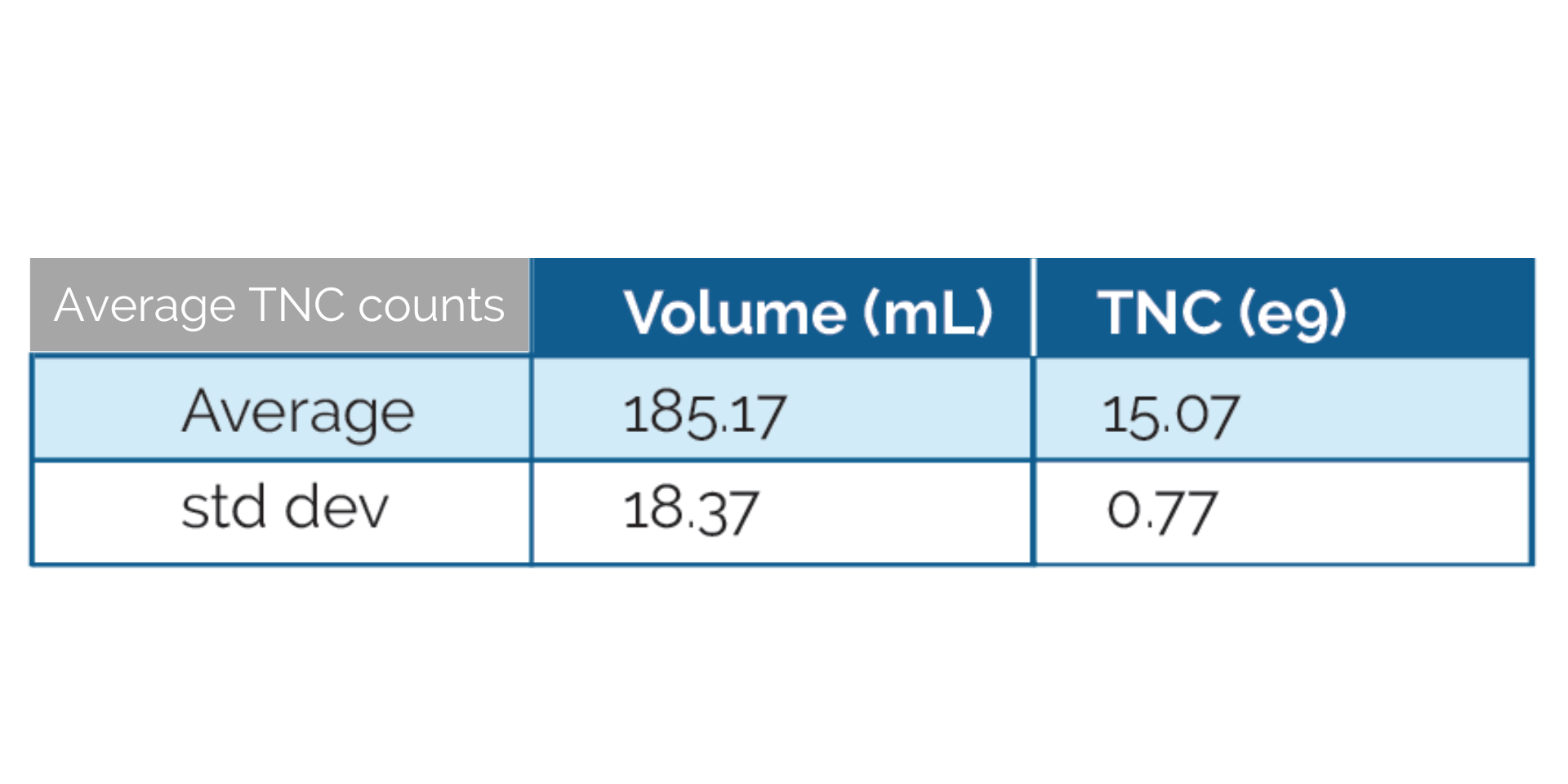
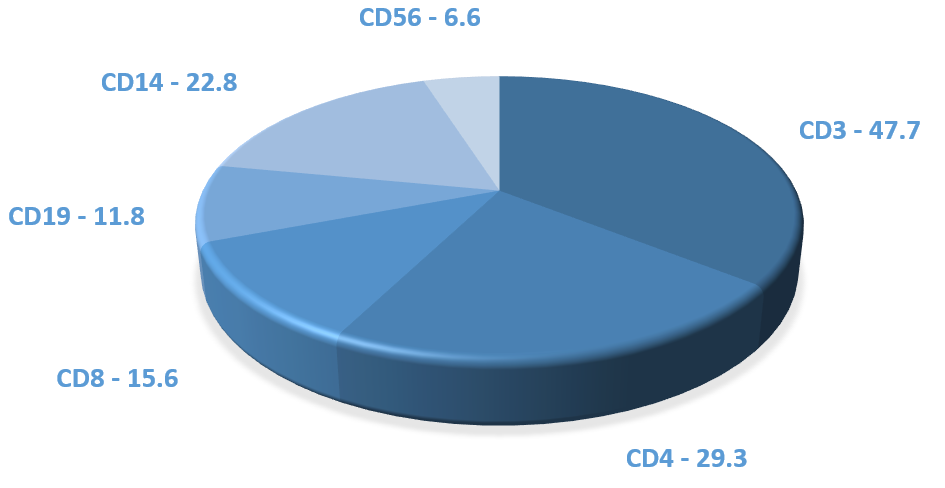
The following are mean percentages of cell populations in a Leukopak:
- T cells – 55%
- Monocytes – 27%
- B cells – 9%
- NK cells – 8%
- CD34+ stem cells – 0.1%
- Granulocytes – 2.4%
We do not guarantee the percentage of cell populations in a Leukopak, as they can vary from donor to donor.
Cells were obtained using Institutional Review Board (IRB) approved consent forms and protocols.
Frozen Leukopaks are collected from IRB consented healthy donors at our FDA-registered CGT Clinics. Using the Spectra Optia® Apheresis System, cell-rich peripheral blood mononuclear cells (PBMCs) are extracted using a continuous flow centrifugation system directly into a sterile collection bag containing an anticoagulant. Besides the PBMCs, which consist of lymphocytes and monocytes, each Leukopak will also contain minimal amounts of granulocytes and red blood cells.
The following are mean percentages of cell populations for a single donor Leukopak prior to cryopreservation:
- T cells – 55%
- Monocytes – 27%
- B cells – 9%
- NK cells – 8%
- CD34+ stem cells – 0.1%
- Granulocytes – 2.4%
We do not guarantee the percentage of cell populations in a Leukopak, as they can vary from donor to donor.

Once collected, Leukopaks are quickly frozen in a medium containing a cryoprotective agent and using a controlled rate freezer to ensure maximum viability.
Total nucleated cell (TNC) counts and viability testing are performed prior to cryopreservation. For optimal cell recovery and viability, we recommend following our “Thawing Protocol” found under the Technical Resources tab. Deviations from this protocol can compromise viability and cell recovery.
For global shipments, we highly recommend shipping Frozen Leukopaks in an LN2 cryoshipper to maintain proper temperatures for the duration of the transit time. Upon arrival, Frozen Leukopaks should be stored in the vapor phase of liquid nitrogen or thawed immediately for use.
A cryopreserved leukopak is a leukopak that has been collected through leukapheresis and then processed and stored using cryopreservation techniques. Cryopreservation involves freezing cells at very low temperatures, typically in a solution containing a cryoprotectant, to preserve their viability and functionality for long-term storage.
Here's how the process generally works:
- Collection: The leukopak is obtained through leukapheresis, a procedure where white blood cells are selectively collected from a donor's blood.
- Processing: After collection, the leukopak is processed to remove any unwanted components and to prepare the white blood cells for cryopreservation. This may involve separating different cell types, washing the cells to remove plasma and platelets, and adding a cryoprotectant solution to minimize damage during freezing.
- Cryopreservation: The processed leukopak is then frozen rapidly to very low temperatures, typically at or below -80 degrees Celsius. This freezing process prevents ice crystal formation within the cells, which can damage cell structures. The cryopreserved leukopak is usually stored in specialized containers, such as cryovials or cryobags, which are designed to maintain stable temperatures during storage.
- Storage: Cryopreserved leukopaks can be stored for extended periods, ranging from months to years, depending on the specific cryopreservation protocol and storage conditions. They are typically stored in ultra-low temperature freezers (-80 degrees Celsius or lower) or in liquid nitrogen tanks to maintain cell viability.
- Thawing and Use: When needed, cryopreserved leukopaks can be thawed and prepared for use in research or clinical applications. Thawing protocols are designed to minimize cell damage and ensure optimal recovery of viable cells. Once thawed, the cells can be used for various purposes, such as cellular therapy, immunological studies, or vaccine development.
Frozen leukopaks are valuable in research and medical applications for several reasons:
- Long-Term Storage: Freezing leukopaks enables long-term storage, allowing researchers and clinicians to maintain a supply of white blood cells for future experiments, therapies, or analyses.
- Flexibility in Timing: Frozen leukopaks can be stored until needed, providing flexibility in experimental or clinical schedules. Researchers can plan experiments or treatments without being constrained by the availability of freshly collected leukopaks.
- Standardization: Frozen leukopaks can be collected and processed under controlled conditions, allowing for standardization across experiments or clinical procedures. This helps ensure consistency and reproducibility of results.
- Cell Banking: Frozen leukopaks can be stored in biorepositories or cell banks, where they serve as a valuable resource for various research studies, drug development, and clinical trials.
- Transportability: Frozen leukopaks can be shipped to different locations for collaborative research or clinical applications, providing access to specialized white blood cell populations regardless of geographic location.
Before use, frozen leukopaks can be thawed and processed to isolate specific cell populations or to prepare them for experimental or therapeutic applications. While freezing can preserve the viability of white blood cells, it's important to note that certain cell types or functions may be affected by the freezing and thawing process, so optimization of protocols may be necessary depending on the intended application.
The cost difference between fresh and cryopreserved leukopaks can vary depending on several factors, including the specific supplier or vendor, the volume of cells, the donor characteristics, and any additional processing or storage requirements. If you would want to know pricing for Frozen Leukopaks for your next project, request a quote from our sales team.
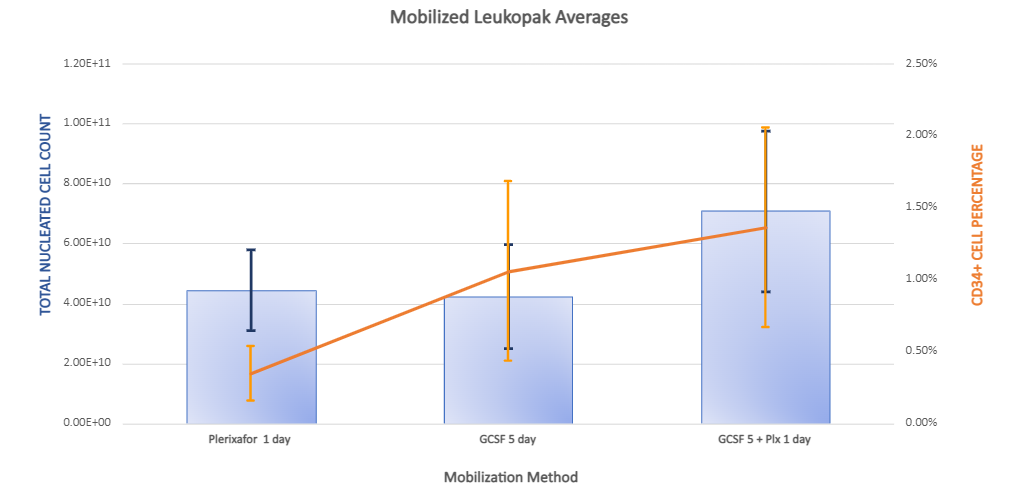
A mobilized leukopak refers to a leukopak that has been collected from a donor after the administration of a mobilizing agent to stimulate the release of white blood cells (leukocytes) from the bone marrow into the bloodstream. Mobilized leukopaks are often collected through leukapheresis procedures and are characterized by a higher concentration of white blood cells compared to leukopaks obtained without mobilization.
The process of mobilizing leukocytes involves the administration of drugs known as mobilizing agents, such as granulocyte-colony stimulating factor (G-CSF). These agents work by stimulating the bone marrow to produce and release white blood cells into the peripheral blood circulation. This results in an increased number of circulating white blood cells, making it easier to collect a higher yield of leukocytes during leukapheresis.
Mobilized leukopaks are commonly used in research, clinical trials, and cell therapy applications where a large number of white blood cells are needed for experimentation or treatment purposes. They may be particularly valuable in studies involving immunology, cellular therapy, vaccine development, and regenerative medicine.
Compared to leukopaks obtained without mobilization, mobilized leukopaks typically yield a higher number of white blood cells, including various cell types such as lymphocytes, monocytes, granulocytes and even hematopoietic stem cells. This increased cell yield can be advantageous for experiments or therapies requiring a larger population of active white blood cells.
Overall, mobilized leukopaks provide researchers and clinicians with a valuable resource for studying immune function, developing new therapies, and advancing our understanding of various diseases and medical conditions.

Human Mobilized Peripheral Blood Leukopaks are collected from healthy IRB consented donors that are injected with granulite stimulating agent and immunostimulant, or a combination of the two which increases circulating leukocytes and stimulates the bone marrow to produce a large number of hematopoietic and progenitor stem cells and mobilizes these cells into the bloodstream, allowing for enriched CD34+ hematopoietic stem cells from a single donor as compared to traditional Leukopaks. Collecting CD34+ stem cells from a single donor eliminates donor-to-donor variation, allows scalability of stem cell studies, and decreases same cell variability. Mobilized Leukopaks are ideal for stem cell studies relating to regenerative medicine, immunotherapy, and transplant therapy.
Mobilized Leukopaks are collected from healthy IRB consented donors that are injected with a combination of two expert grade stimulating factors the day before collection, which stimulates the bone marrow to produce hematopoietic stem cells and mobilizes them into the bloodstream, prior to first day of collection. Mobilized mononuclear cells are collected by a trained technician using the Spectra Optia® Apheresis System one and two days post injection. After collection, mobilized Leukopaks are checked by flow analysis to verify the percentage of granulocytes, mononuclear cells, CD34+ cells, and cell viability.
- 2x10e TNCs/1 bag
- Enriched PBMCs
- Enriched CD34+ cells
- Low hematocrit
- Low granulocytes
- Cells were obtained using Institutional Review Board (IRB) approved consent forms and protocols.
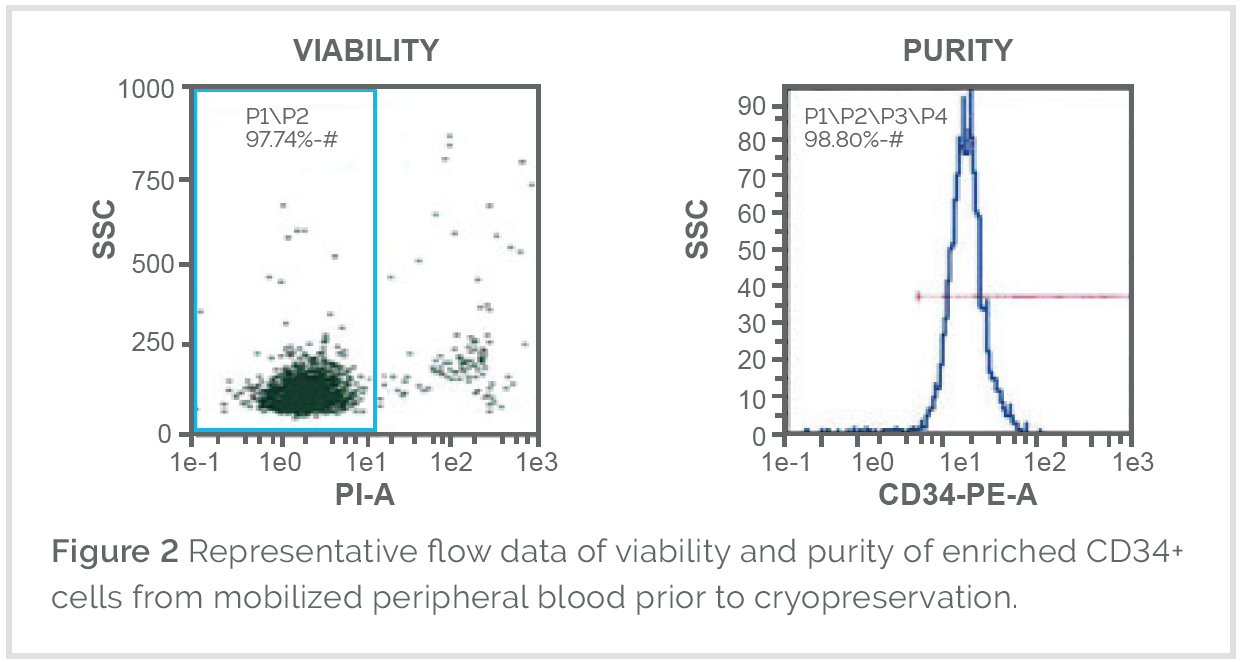
Clinical Fresh Leukopak products contain a high concentration of all major lymphocyte populations (T Cells, B Cells, NK Cells, and monocytes) and are GTP/GMP compliant products for cell-based therapy as well as used as the starting material for further manufacturing in allogeneic cell therapies. Our donors follow FDA 21 CFR 1271 guidelines and our productions follow enhanced quality training and are subjected to CMNC protocol in our FDA-registered CGT clinics. Depending on the unique requirements and expectations, CGT Global can customize the donor selection process, infectious disease screening and screening timepoints along with the collection to meet clients needs as well as international regulatory requirements.

A GMP (Good Manufacturing Practice) compliant leukopak refers to a leukopak that has been collected, processed, and stored in accordance with strict regulatory guidelines and quality standards outlined by regulatory authorities, such as the Food and Drug Administration (FDA). GMP regulations are designed to ensure the quality, safety, and efficacy of pharmaceuticals, biological products, and medical devices.
For leukopaks to be considered GMP compliant, the entire process, from donor screening to leukapheresis, processing, storage, and distribution, must adhere to GMP principles. This includes implementing standardized procedures, maintaining appropriate facilities and equipment, establishing quality control measures, and documenting all aspects of the collection and handling process.
Key aspects of GMP compliance for leukopaks may include:
- Donor Screening: Ensuring that donors undergo thorough screening procedures to assess eligibility and minimize the risk of transmitting infectious diseases or other adverse events.
- Aseptic Collection and Processing: Performing leukapheresis and subsequent processing steps under sterile conditions to prevent contamination of the leukopak.
- Quality Control: Implementing quality control measures to monitor the integrity, purity of leukopaks, and potency of the leukopak throughout the collection, processing, and storage process.
- Documentation and Recordkeeping: Maintaining detailed records of donor information, collection procedures, processing protocols, and storage conditions to facilitate traceability and accountability.
- Facility and Equipment Compliance: Ensuring that facilities and equipment used for leukopak collection, processing, and storage meet GMP standards and are regularly inspected and maintained.
- Staff Training and Qualification: Providing training and education to personnel involved in leukopak collection and processing to ensure competency and compliance with GMP requirements.
GMP compliant leukopaks are often required for clinical research, investigational drug development, and cell therapy applications intended for use in humans. By adhering to GMP standards, organizations can demonstrate the quality and reliability of leukopaks, ultimately enhancing patient safety and regulatory compliance.
Customization

Customize Your Leukopak
- Fresh or Frozen
- Raw material or isolated cells
- Customized IRB or ICF
- Matched cell types and custom cell counts
- Donor demographics/characteristics
- Shipping, packaging, courier
- Additional infectious disease screening (CMV, etc.)
- Much more....
Additional Services

Obtaining a specific primary cell population from human tissue can be time-consuming and costly. If not done quickly and properly, cell viability and recovery can be compromised, creating new challenges to overcome and delays in your research.
To provide high-quality cells to scientists and keep their research moving forward, each CGT Clinic houses an on-site state-of-the-art laboratory, providing an end-to-end service whereby cells are processed, isolated, and cryopreserved or shipped out fresh immediately after collection from a donor. This end-to-end service provides accountability and traceability for each product that leaves our facilities. Additionally, all methods involved in tissue collection, cell processing, cell isolation, and analysis follow strict guidelines and quality standards ensuring consistent, compliant, viable, and pure cells with guaranteed cell counts.
We work with each client to make sure they receive the right primary cells for their project. Therefore, each step of the process is customizable to suit the specific needs of the client, starting with the right donor with specific attributes to isolating and analyzing a specialized cell type.
CGT Global offers on-site equipment validation support for our clients looking to verify that their devices meet FDA and GMP standards. Our testing process ensures full compliance and optimization of lab operations prior to product distribution. Bring your device to our state-of-the-art labs to ascertain that your equipment performs all of its intended functions with resolution and fidelity.







